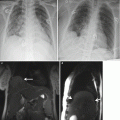
Localizer T2 SSFSE
T2 2D SSFSE
T2 2D SSFSE
T2 2D
FS FSE
T1 2D
SPGR Dixon
DWI b50, 600
T1 3D SPGR FS
Plane of acquisition
Axial, coronal, sagittal
Axial
Coronal
Axial
Axial
Coronal
Axial
Matrix
256 × 128
256 × 160
256 × 192
320 × 160
256 × 160
128 × 128
256 × 160
TR/TE (ms)
828/63
1000/60
1000/60
2200/87
180/4.36 (in), 2.06 (out)
4100/64
3.63/1.73
Flip angle (°)
90 to >150–180
90 to >150–180
90 to >150–180
90 to >150–180
80
90
15
ST/SG (mm)
5.0/10.0
4.0/5.0
4.0/5.0
7.0/9.0
3.0/7.5
8.0/10.0
2.0/1.6
NEX
0.6
0.6
0.6
1
1
1 (b50)
3 (b600)
0.7
RBW
651
488
488
122.1
244
1953
300
Phase direction
A to P, R to L, A to P
A to P
A to P
A to P
A to P
R to L
A to P
Echo train length
76
96
115
160
160
128 (b50) 384 (b600)
112
FOV (mm)
650
350–400
350–400
350–400
350–400
350–400
350–400
Respiration
BH
BH
BH
BH
BH
BH
BH
Fat saturation
–
–
–
–
–
–
+
Concatenationa
1
2–3
2
2–3
2
1–2
1
Parallel imaging (acceleration factor)
–
–
–
–
–
–
1.7/2.1
Table 21.2
Parameters for pancreas and biliary imaging on 1.5 T MR imaging scanner
Localizer T2 SSFSE | T2 2D SSFSE | T2 2D SSFSE | T1 2D SPGR dixon | DWI b50, 600 | T1 3D SPGR FS | MRCP 2D thick slab | MRCP 3D thin slab | |
|---|---|---|---|---|---|---|---|---|
Plane of acquisition | Axial, coronal, sagittal | Axial | Coronal | Axial | Coronal | Axial | Coronal | Coronal |
Matrix | 256 × 128 | 256 × 160 | 256 × 192 | 256 × 160 | 128 × 128 | 256 × 160 | 384 × 256 | 256 × 256 |
TR/TE (ms) | 1500/60 | 1000/62 | 1000/60 | 180/4.36 (in), 2.06 (out) | 4100/64 | 3.65/1.75 | 2284/600 | 3750/400 |
Flip angle (°) | 90 to >150–180 | 90 to >150–180 | 90 to >150–180 | 80 | 90 | 15 | 90 | 90 |
ST/SG (mm) | 7.0/12.0 | 4.0/5.0 | 4.0/5.0 | 6.0/7.5 | 8.0/10.0 | 3.2/1.6 | 50/51 | 1.4/0.7 |
NEX | 0.6 | 0.6 | 0.6 | 1 | 1.3 | 0.7 | 0.6 | 1 |
RBW | 651S | 390 | 390 | 488 | 1953 | 244 | 300 | |
Phase direction | A to P, R to L, A to P | A to P | A to P | A to P | R to L | A to P | A to P | A to P |
Echo train length | 76 | 96 | 115 | 160 | 128 (b50) 384 (b600) | 112 | 154 | 256 |
FOV (mm) | 450–500 | 350–400 | 350–400 | 350–400 | 350–400 | 350–400 | 350–400 | 350–400 |
Respiration | BH | BH | BH | BH | BH | BH | BH | BH |
Fat saturation | – | – | – | – | – | + | + | + |
Concatenationa | 1 | 2–3 | 2 | 2 | 1–2 | 1 | 5 | 1 |
Parallel imaging (acceleration factor) | – | – | – | – | – | 1.7/2.1 | – | 3 |
21.3 Liver
Physiological changes that accompany pregnancy should not be confused with liver dysfunction (Table 21.3). Increase in serum alkaline phosphatase levels up to three to four times above normal, due to placental production, and mild increase in fibrinogen and ceruloplasmin levels are typical for normal pregnancy, as well as changes in serum alpha-fetoprotein levels. Mild decrease in factors participating in fibrinolysis – antithrombin III and protein S – is also typical, although there is no change in prothrombin time.
Table 21.3
Physiologic changes of pregnancy
Increase |
Serum alkaline phosphatase (from placental production) |
Clotting factors: factor I (fibrinogen), factors VII, VIII, X, and XII, Von Willebrand factor, protein S-binding protein, plasminogen activator inhibitor (PAI)-1 and PAI-2 (PAI-2 is produced by the placenta) |
Ceruloplasmin |
Transferrin |
Alpha-fetoprotein |
Blood volume, heart rate, cardiac output |
Decrease |
Fibrinolytic factors: antithrombin III and protein S |
Uric acid |
Gallbladder contractility |
Albumin and total serum protein (due to dilution effect from increased blood volume) |
Hemoglobin (due to dilution effect from increased blood volume) |
Systemic vascular resistance |
Blood pressure |
No change |
Liver transaminases: aspartate aminotransferase (AST), alanine aminotransferase (ALT) |
γ-Glutamyl transferase (GGT) |
Bilirubin |
Prothrombin time (PT) |
Platelet count (may decline slightly due to dilution effect from increased blood volume) |
Serum amylase |
Three to 5 % of pregnancies are complicated by truly abnormal liver function tests, caused by a variety of hepatobiliary diseases, including infectious, autoimmune, neoplastic, and inherited causes (Table 21.4).
Table 21.4
Causes of hepatobiliary disease in pregnancy
Unique to pregnancy |
Hyperemesis gravidarum |
Intrahepatic cholestasis of pregnancy |
Acute fatty liver of pregnancy |
Preeclampsia |
HELLP syndrome (hemolysis, elevated liver enzyme levels, low platelet count) |
Coincidental with pregnancy |
Gallstone-associated disease: |
Cholelithiasis |
Choledocholithiasis |
Cholecystitis |
Ascending cholangitis |
Liver abscess |
Acute pancreatitis |
Acute viral hepatitis |
Budd-Chiari syndrome |
Portal vein thrombosis |
Focal lesion (hepatic adenoma, HCC) |
Preexisting disease |
Chronic viral hepatitis B and C |
Cirrhosis and portal hypertension |
Autoimmune hepatitis |
Primary biliary cirrhosis |
Primary sclerosing cholangitis |
Wilson’s disease |
Neoplasia (hemangioma, FNH, hepatic adenoma) |
Although uncommon, any liver disease can occur coincidentally in the pregnant patient, as well as pregnancy may occur in a patient with preexisting underlying chronic liver disease. However, most liver dysfunction in pregnancy is pregnancy induced and due to one of the five liver diseases unique to the pregnant state: hyperemesis gravidarum, intrahepatic cholestasis of pregnancy, acute fatty liver of pregnancy, preeclampsia/eclampsia, and the Hemolysis, Elevated Liver enzymes and Low Platelets (HELLP) syndrome. These conditions are complications of the pregnancy itself, and all have a characteristic timing in relation to the length of the pregnancy: hyperemesis gravidarum in the first trimester, intrahepatic cholestasis of pregnancy in the second half of pregnancy, and the remaining three in the third trimester (Table 21.5).
Table 21.5
Hepatobiliary disorders unique to pregnancy
Disorder | Gestational status | Prevalence | Symptoms | Outcome | Definite treatment |
|---|---|---|---|---|---|
Hyperemesis gravidarum | 1st trimester Primiparous | 0.3 % | Intractable nausea and vomiting | Benign for mother and fetus | Supportive, total parenteral nutrition |
Intrahepatic cholestasis of pregnancy | 2nd trimester | 0.7 % | Pruritus | Benign for mother and fetus; increased gallstone disease | Ursodiol; delivery |
Acute fatty liver of pregnancy | 3rd trimester Primiparous 50 % have eclampsia | 1:10,000–15,000 | Nausea, vomiting, right upper quadrant or epigastric pain, malaise; quick progression to fulminant hepatic failure, DIC | Maternal mortality 20 %; fetal mortality up to 45 % | Delivery Test for LCHAD |
Preeclampsia | Late 2nd and 3rd trimester Multiparous Multifetal | 5 % | High blood pressure, proteinuria, edema, seizures, renal failure, pulmonary edema | Maternal mortality 1 %; prematurity; fetal death 30 % | Delivery |
HELLP syndrome | Late 2nd and 3rd trimester, postpartum 12 % have eclampsia Multiparous Multifetal | 0.5 % | Abdominal pain, seizures, renal failure, pulmonary edema, liver hematoma, and rupture | 60 % maternal mortality when hepatic rupture; fetal death 30 % | Delivery |
21.3.1 Hepatic Conditions Unique to Pregnancy
In general, MRI in patients with hyperemesis gravidarum and intrahepatic cholestasis of pregnancy does not have a role other than to exclude possible differential diagnoses that may cause hepatic dysfunction.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree