Postprocedure and follow-up care
Following the procedure, longitudinal care of the patient should be provided as described elsewhere in this text (e.g., see Chapter 4: “Catheter fixation and drainage” and “Catheter removal”). As elsewhere, catheter management should be continued, including daily rounds under the supervision of the interventional service, until the process has resolved and the catheter has been discontinued. The interventional service may wish to schedule the patient for a follow-up visit in the outpatient IR clinic two to four weeks after catheter removal to evaluate the outcome and assure appropriate management of any complication or recurrence.
Complications
The main risks related to aspiration and drainage procedures include bleeding, infection, pain, and incomplete treatment with recurrence. Significant complications should very seldom be encountered. If a drain is placed adjacent to a bony structure, consideration should be given to the potential for catheter kinking or compression. Compression of a catheter against periosteum or within a muscle belly can be painful and should be avoided where possible.
Conclusions
The primary advantages of percutaneous aspiration and drainage with imaging guidance in the MSK system and soft tissues are less tissue trauma, shortened recovery time, and reduced cost in comparison to an open surgical procedure designed to accomplish the same objective.
Biopsy of lymph nodes and soft tissue masses
Introduction
Evaluation of the child with a soft tissue mass begins with consideration of the patient’s age, the location of the mass, and the clinical history, including any known underlying predisposing conditions. The location and tissue origin of the mass can often be established with imaging. Plain radiography can be useful to examine the relationship of the mass to osseous structures and to demonstrate the presence of soft tissue calcifications. Ultrasound is perhaps most useful for characterization of soft tissue masses or nodules and to differentiate solid from cystic masses. Ultrasound offers the flexibility of a vascular study without requiring contrast administration, and can yield a great deal of physiologic information. Despite its cost and the frequent requirement for sedation or anesthesia, MRI remains the most versatile modality for analysis of a soft tissue mass. It provides excellent tissue contrast, ability to image deeply situated and large masses, can demonstrate invasion of deep tissue planes and involvement of major neurovascular bundles, and contributes significantly to the search for tumor extension and metastasis. Multiplanar reconstruction can facilitate determination of the tissue of origin, and arterial and venous imaging can help determine the vascularity of the lesion. Other modalities may offer specific advantages in limited situations, such as the evaluation of myositis ossificans with CT, and the further characterization of vascular supply, drainage, and spaces with angiography and venography.
Once a mass has been characterized to the degree possible with imaging, a few abnormal masses may be so uniquely identified that further differentiation is not necessary. For the rest, cellular or histopathologic evaluation is necessary before a final diagnosis is possible.
Indications
The differential for a palpable mass in the soft tissues includes pseudotumors, neoplastic tumors, and vascular malformations (Table 7.4). Pseudotumors in general do not require biopsy. Most can be identified by imaging characteristics in light of the patient’s age, sex, location, and clinical history. Some, such as axillary breast tissue, post-traumatic lesions, and granulomata, simply need to be identified and left alone. Others, such as hematoma, cellulitis, and abscess, may require aspiration, drainage, or systemic therapy. Symptomatic periarticular cysts may benefit from aspiration and, if recurrent, steroid instillation. Vascular neoplasms and malformations, which are the most common cause of soft tissue masses in children, are considered in detail in Chapter 8.
Possible malignancy
Evaluate for recurrence
Differentiate between metastasis and infection
Non-vascular neoplasms include fat-containing or adipocytic tumors (e.g., lipoma and liposarcoma), fibrous mesenchymal tumors (including such entities as nodular fasciitis and myositis ossificans, as well as infantile and adult-type fibrosarcoma), fibrohistiocytic tumors (including pigmented villonodular synovitis, giant cell tumor of the tendon sheath, dermatofibrosarcoma protuberans, and malignant fibrous histiocytoma), smooth muscle and skeletal muscle tumors (e.g., leiomyoma and rhabdomyosarcoma), a variety of soft tissue sarcomas that may be of uncertain origin, lymphoma, and tumors of neurogenic origin (e.g, schwannoma, neurofibroma, and malignant peripheral nerve sheath tumors) that may have a soft tissue presentation.
In some patients, a history of prior malignancy or predisposing factors, such as neurofibromatosis type 1, narrow the differential diagnosis. When the imaging characteristics of a previously treated malignancy are known, such as an FDG-avid lymphoma, repeat imaging with the same technique may discriminate between recurrent tumor and a benign process, and may obviate biopsy. However, the majority of abnormal nodules and soft tissue masses in children should be biopsied. Biopsy can also be useful to determine the causative organism in suspected infection or infection unresponsive to empiric therapy.
The only absolute contraindication to biopsy in the soft tissues is an arterial mass. The vascularity of each lesion should be known prior to a biopsy procedure. Biopsy should not be performed through overlying tissues that are infected, and a predisposition to bleeding should be corrected to the degree possible prior to an invasive procedure.
Technique
Equipment
The necessary equipment and standard technique for soft tissue biopsy is no different from that used in biopsies of the chest, abdomen, or pelvis (see Table 4.16).
Patient preparation
Unlike procedures in the osseous skeleton, biopsies performed in the soft tissues may be safely and comfortably completed under sedation with generous local anesthesia. There are exceptions, including (1) small lesions that are deeply situated, which may require paralysis of the uncooperative patient, (2) large masses, especially those that are hypervascular, adjacent to vital structures such as the airway, where hemorrhage may create or augment mass effect and where airway control or volume management by an anesthesiologist may be a beneficial precaution, and (3) multiple procedures planned in the same encounter, where the total procedure time is likely to exceed an hour. However, in many practices general anesthesia is preferred.
Even though US is often the most useful modality for image guidance during a biopsy procedure, it is important to review available cross-sectional imaging to localize the lesion and correlate with its US appearance, determine its suitability for biopsy, and identify any adjacent vital structures and important anatomic relationships. If there is uncertainty regarding the conspicuity of a lesion on US that has already been defined with CT, PET-CT, or MR, it is a simple matter to bring US into the CT suite so that the lesion can be localized with CT and the biopsy completed with US or CT as necessary. Regardless of which imaging modality is selected for biopsy guidance of a suspected malignancy, it is essential to sample the most representative tissue within the lesion and, where multiple lesions are present, to determine which are most metabolically active and the lesions with the safest and easiest access. We find that preprocedural PET-CT will improve target selection in >20% of cases, depending on the pathology.
Standard technique
The technique for biopsy is similar to other sites (as outlined in Chapter 4). A coaxial approach facilitates rapid acquisition of multiple specimens within each quadrant of the mass. In larger masses this can be combined with a tandem needle approach (see “Coaxial and tandem needle techniques,” in Chapter 4), leaving the guiding tandem needles in place to assist the interventionalist in identifying which quadrants have already been sampled and which remain.
The results from percutaneous lymph node biopsy are generally not as rewarding as those from other organs. Often, several core biopsies are needed in several locations within a node in order to get an accurate diagnostic result. Otherwise, percutaneous biopsy of abdominal and pelvic nodes may be performed with imaging guidance following PET-CT. In cases of suspected lymphoma, percutaneous lymph node biopsy is usually performed with US or CT guidance. Nodes and small nodular lesions may be mobile and easily pushed away from the biopsy needle. It may be useful to stabilize the nodule: first, by introducing the tip of a semi-automated needle into the margin of the node prior to firing and, second, by “propping” the nodule opposite the needle entry site with the operator’s fingertips or other suitable support. An end-cutting needle is helpful when biopsy of a small lesion is performed.
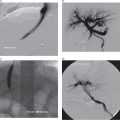
The approach to diagnosis of mass lesions in children is entirely different than in lymph nodes (Table 7.5). Percutaneous image-guided biopsy is generally the method of choice for virtually all mass lesions of childhood (Figure 7.2). Surprisingly, it is often most difficult to obtain a pathologic diagnosis in patients with very large lesions (greater than 8 to 10 cm in diameter) compared to smaller (<2 cm diameter) lesions. While the reasons for this are not entirely clear, they may include presence of extensive central necrosis, mucinoid changes, and intralesional diversity. Thus in order to maximize the diagnostic utility of the biopsy, we suggest using ≥16-gauge core biopsy needles to sample multiple quadrants within the mass, taking many samples (i.e., greater than five), and concentrating sampling in target regions where imaging suggests viability and high metabolic activity. In cystic (non-vascular) lesions, focus should be on the margin of the lesion, especially any focal nodular component, and any component that demonstrates vascularity on Doppler imaging.
Sedation/general anesthesia
Ultrasound (or other modality) localization of skin entry site. Mark entry site with washable ink
Sterile preparation and draping
Ultrasound (or other modality) guided needle placement into mass lesion
Sample all four quadrants of the lesion with >16-gauge biopsy needle if possible
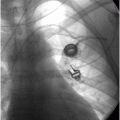
Five-year-old female with enlarging blue-colored soft tissue mass of the forehead and glabella.
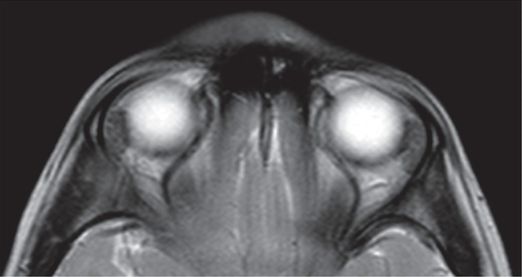
T2 axial MRI at level of orbits showing homogeneous subcutaneous mass.
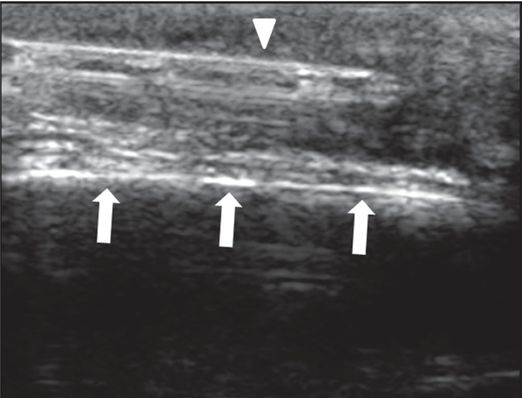
Ultrasound-guided 18-gauge BioPince® core needle (arrowhead) specimens were obtained in the mass superficial to the calvaria (arrows), leading to the diagnosis of lymphoblastic lymphoma.
Specimen handling and the usefulness of preliminary intraprocedural histopathologic evaluation (frozen section or touch-prep) have been discussed in detail in Chapter 4. It is worthwhile to repeat here that consultation, prior to the procedure, between the interventional radiologist, oncologic orthopedic surgeon, and pathologist, is extremely important to define the preferred needle route so that only the structures intended to be removed are traversed, so that the needle tract can be removed completely (if the surgeon prefers because of the risk of tract seeding), and to plan for frozen sections or other related pathology needs.
Postprocedure and follow-up care
Following the biopsy, immediate postprocedure imaging should be performed to exclude procedural complications, such as hemorrhage or injury of adjacent vital structures. If the procedure is performed with US, anatomic imaging and color Doppler of the lesion and adjacent structures may be sufficient. If the target location is in a particularly vulnerable area, CT sections through the area of interest can provide a more global assessment at the cost of a relatively small radiation dose. In most cases, the dermatotomy can be approximated with Dermabond® and covered with a transparent dressing, and no additional follow-up should be required. Postprocedural hemoglobin and hematocrit are rarely necessary, unless there is a bleeding complication. The patient and family should be informed of the possibility for postbiopsy bleeding, infection, or pain, and contact telephone numbers for the interventionalist should be given to the family. If there is a high likelihood of postprocedure discomfort, a prescription for appropriate analgesics should also be provided.
Complications
Bleeding is the most common complication of biopsy procedures in the soft tissues. When it occurs at the percutaneous entry site, it is usually readily observed and easily managed with manual compression. Hemorrhage in the deep soft tissues, the mass, or from surrounding vessels may be subtle, and in rare cases, may lead to hypovolemic shock or compression of adjacent vital structures. If there is concern for a bleed, US including color Doppler or CT of the area is recommended.
Other complications related to vascular injury during a soft tissue biopsy, such as pseudoaneurysm, arteriovenous fistula, and tract seeding are possible but rare.
Conclusions
Core biopsy of soft tissue masses, nodules, and lymph nodes should be performed to answer specific clinical questions where the answers are likely to materially affect the management of the patient. Communication between the interventionalist, clinical service, and pathologist before the biopsy should be directed to planning and executing the acquisition of sufficient representative tissue so that clinical questions are addressed in order of priority. Issues related to specimen handling, transport, and processing should be clarified with the pathology service before the procedure begins. If additional procedures are needed, such as insertion of a central venous catheter, it is ideal to perform them under the same anesthetic. Preprocedural imaging for lesion characterization and localization as well as selection of the optimal lesion to sample and the optimal level and route for sampling will help ensure a high rate of safe and successful procedures, with a low rate of complications and significantly lower cost than open surgical alternatives.
Foreign body retrieval
Introduction
When a potentially dangerous object is introduced into the skin or soft tissues from the outside, it places the patient at risk for injury to vital structures, infection, pain, or dysfunction. (Management of intravascular foreign bodies is described in Chapter 2, and retrieval of ingested foreign bodies is covered in Chapter 3.) These foreign bodies may be composed of a variety of materials, such as metal, glass, ceramic, plastic, mineral (e.g., rock shards and gravel), pencil graphite, or organic (e.g., bone, fish spines, teeth, wood splinters, and thorns). In addition, remnants of shoes, socks, or clothing (such as leather, cloth, or rubber) may accompany a foreign body into the wound. The entry wound and mechanism of injury may localize the foreign body in general terms, but the angle, depth of penetration, and degree of fragmentation are often uncertain. So it is that surgical removal begins with sharp and blunt exploration that may be protracted and involve considerably more trauma to tissue than originally involved in the wound.
Conventionally, foreign bodies in the soft tissues are localized with underpenetrated plain radiography first, then the radiographs are used by emergency department physicians or surgeons to guide surgical exploration and removal. For suspected objects that are difficult to identify or localize with plain radiography, US or as a last resort, CT may be used. Objects that are located near vital structures such as nerves and vessels may be left in place if they are not symptomatic or infected. Image-guided foreign body removal allows accurate localization of the foreign body and removal through a small incision with minimal additional trauma or untoward effect.
Indications
Any foreign body that causes pain should be removed if possible (Table 7.6). Removal of a foreign body in the soft tissues can be attempted for virtually any object that can be visualized with US. While objects are easiest to remove within the first 24 hours, even objects that have been embedded much longer, with accompanying inflammation, induration, suppuration, scarring, or granulation tissue, may be removed using these techniques. Many objects that are not detected on plain radiographs can be localized with US. In addition to providing information about the depth, size, and shape of the foreign object, US can also define its relationship to important anatomic structures such as nerves, vessels, tendons, ligaments, joints, and bones. Any foreign object that can be visualized should be removed under direct visualization, including objects embedded in the nose and ears. An ophthalmologist should be consulted for removal of foreign bodies in the cornea of the eye. Similarly, aspirated foreign bodies are usually removed by a pulmonologist under endoscopic visualization. Inert foreign bodies such as bullet or glass fragments that do not threaten vital structures, are not infected, and are asymptomatic may not need to be removed.
Technique
Equipment
High-quality US with good near-field resolution is very helpful for this procedure. A 10 ml or 20 ml syringe filled with sterile saline, water, or thinned US gel attached to a 16- or 18-gauge angiocatheter can be used to “float” the foreign body once it is localized. A #11 scalpel blade, splinter forceps, and a mosquito or Kelly forceps are usually sufficient to remove most foreign bodies in the soft tissues (Table 7.7).
Patient preparation
If a foreign body is suspected in a puncture wound, and cannot be removed under direct visualization, its presence should be confirmed with imaging. If the foreign body can be clearly identified with US, no other imaging is required. If US is equivocal, underpenetrated plain radiography or CT may help to confirm or further characterize the target object(s).
The puncture site may be prepared with a J-Tip® (pressurized intradermal infusion of 1% buffered lidocaine using CO2) because of its immediate time to action (Figure 7.3). The site is then prepared and draped in sterile fashion. Infiltration with local anesthetic or regional nerve blocks may be very useful where appropriate.
Skin anesthetic. (a,b) Use of J-Tip®.
Standard technique
When necessary, the child may be sedated or anesthetized. If the procedure is performed under sedation or local anesthesia only, the site is anesthetized with a J-Tip®and 1% buffered lidocaine infiltrated into the tract with a 30-gauge needle. The foreign body is visualized en face, to the degree possible. If the object’s proximity to the skin surface makes visualization difficult, a stand-off pad or thick layer of gel may assist imaging. A stab wound is created with the scalpel blade, and carried down to the superficial margin of the foreign body (Table 7.8). The saline-filled angiocatheter or needle is advanced through the wound under US guidance, and saline is injected around the object. This has the effect of “floating” the object free from the surrounding soft tissue, and increasing its conspicuity to US imaging. Forceps are then advanced through the wound/incision, under US visualization, until they come in contact with the foreign object. The foreign body is grasped and removed (Figure 7.4).
Preprocedural US to identify foreign body and identify skin entry site
Mark entry site with washable ink
Sedation/general anesthesia as needed
Local anesthesia with J-Tip®
Sterile preparation and draping
Tract anesthesia using 1% lidocaine
Skin incision
Identify foreign body and inject saline around it
Forceps removal of foreign body under US guidance
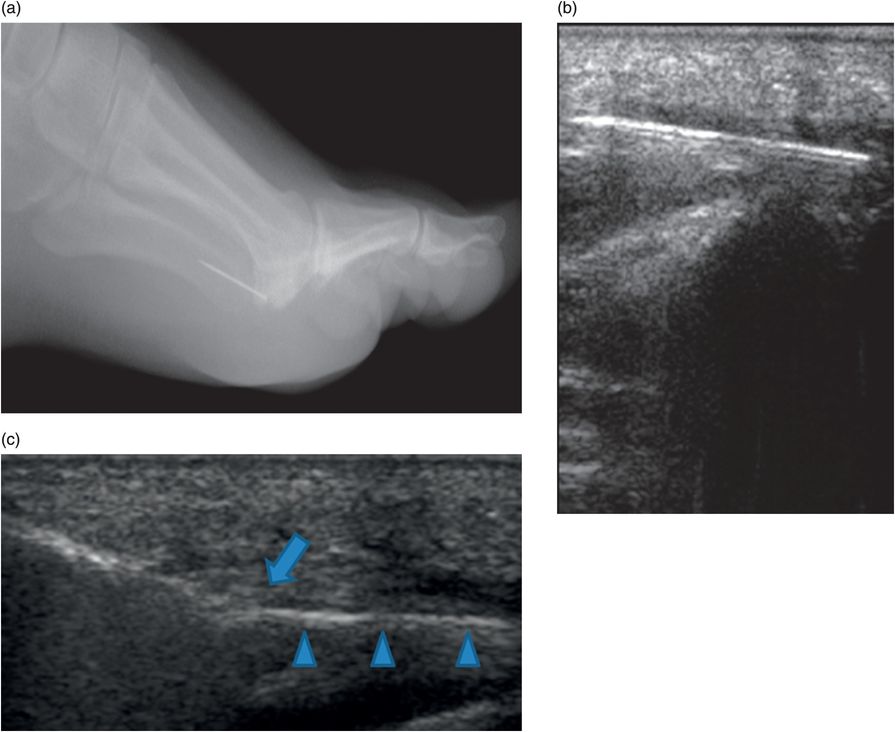
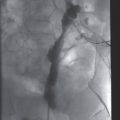
Thirteen-year-old female with sewing needle fragment in plantar soft tissues of the foot with swelling and erythema. (a,b) Lateral foot radiograph and US show needle fragment. (c) Ultrasound-guided forceps retrieval. Note: tip of forceps (arrow) grasping needle fragment (arrowheads).
Technical variations
In the unusual case where a foreign body is visualized on a plain radiograph or by CT but is not apparent on US, it is possible to use biplane fluoroscopy to guide forceps to the object. Failing this, a localizing needle may be placed adjacent to the foreign body to assist surgical exploration and retrieval.
Postprocedure and follow-up care
Once the foreign body, including accessible fragments, has been removed, the puncture wound is again irrigated with saline or sterile water, hemostasis is achieved, and the wound is left to heal by secondary intention. It should be dressed with a loose gauze bandage. If a skin incision has been created distant from the original puncture wound, this may be closed with suture or Dermabond® if necessary. The patient and family should be instructed to return if any signs or symptoms of infection are noted. In this case, further evaluation for a retained foreign body should be undertaken.
Tetanus toxoid prophylaxis, and tetanus immune globulin, should be considered unless the child’s vaccination status is up to date. Prophylactic antibiotics are usually not necessary, but may be indicated in the case of a human bite wound, or when the foreign body involves bone or joint penetration.
Complications
Infection is the most common complication of a foreign body, although the rate of infection from non-bite wounds is small. The rate is highest in patients with a compromised immune status, or when wounds are visibly contaminated, large, jagged, or deep. Occasionally foreign bodies will be missed, or significant fragments will be left behind that may later cause infection or pain, or may migrate to a more dangerous position.
Conclusions
Wounds with a neglected foreign body are a common cause of litigation. Conventional surgical methods of removal may cause additional tissue trauma due to the difficulty of localizing the object during exploration. An image-guided approach allows detailed evaluation of the foreign body and its anatomic context, and real-time visualization of the target object during acquisition and retrieval. This reduces the need for exploration and associated tissue injury. Although antibiotic prophylaxis is rarely necessary, there should be a low threshold for tetanus prophylaxis, as the incidence of missed tetanus prophylaxis after penetrating injuries is as high as 6%.
Chemodenervation of the salivary glands
Introduction
Excessive drooling occurs when normal swallowing is impaired, with or without elevated levels of saliva production. This occurs most frequently in the setting of cerebral palsy, but may occur due to a variety of chronic and less frequently, acute causes. Common contributing factors include inability to sense saliva pooling in the mouth and incoordination of swallowing. The inability to handle oral secretions may lead to silent aspiration, recurrent pneumonia, reactive airways disease, and other aspiration-related events. Chronically pooled secretions also lead to poor oral hygiene, gingivitis, and halitosis. Constant drooling leads to excoriation of the skin, soiled clothing, poor public acceptance, and difficulty for the caregiver. Successful control of these secretions is often life-changing for the patient and family.
For the child with excessive drooling, it is first important to establish the cause. Treatable acute causes should be identified and treated. Some patients with behavioral or neuromuscular disability may respond to behavioral modification and occupational therapy. In some circumstances, medical therapy with anticholinergic drugs such as atropine sulfate or glycopyrrolate may be beneficial in decreasing the activity of acetylcholine muscarinic receptors, and therefore decreasing saliva production. Patients who do not respond to these behavioral and medical treatments and who are at risk for aspiration-related complications may be good candidates for chemical denervation of the salivary glands.
Indications
Any child with excessive drooling who has experienced recurrent aspiration pneumonia or who has been diagnosed with other aspiration-related disorders, such as reactive airways disease with impairment of pulmonary function, is likely to benefit from this procedure. Those who have choking or severe coughing, drooling at night, poor oral hygiene or halitosis, or drooling-related skin irritation may also be reasonable candidates. Often these are children with cerebral palsy, but children with mental retardation, stroke, and degenerative neuromuscular disorders also may have chronically excessive drooling. Acute causes of excessive drooling, such as poisoning (e.g., mercury, some pesticides), infection (e.g., strep throat, tonsillitis, peritonsillar or retropharyngeal abscess) or tumors that compress the upper digestive tract are not appropriate candidates. The only contraindication to this procedure is a prior history of allergic reaction to botulinum toxin A, which may present as hives, difficulty breathing, or swelling of the face, lips, tongue, or throat. Patients with hypotonia or weakness of muscles of breathing or swallowing, may be at increased risk of life-threatening complications related to injection of botulinum toxin A. Just as with any other percutaneous procedure, injections should not be given through broken or infected skin. It is also important to inquire into the patient’s surgical history, as occasionally these patients may have undergone resection of one or more glands (usually, the submandibular glands). Knowing this will save time searching for them – or worse, treating where the glands aren’t.
In healthy individuals, saliva production is estimated to be between 0.75 to 1.5 liters per day. During sleep the volume drops to near zero. The submandibular glands are responsible for about 70 to 75% of the total saliva production, while the parotid produces 20 to 25%. The other salivary glands produce very little. The parotid gland production of saliva is stimulated when eating, therefore, at baseline, most saliva is from the submandibular glands.
Technique
Equipment
The only equipment required for this procedure are 1-ml syringes, 1.5-inch needles, botulinum toxin A, and a high-frequency linear transducer (15 to 7 MHz) with a small footprint, which is easiest to manipulate in this region (Table 7.9). We use Botox® A. This is an off-label use of this medication: it is not FDA approved for this purpose at the time of writing. The medication should be reconstituted with saline according to the manufacturer’s directions. Other strains of Clostridium botulinum (e.g., B, E, and F) should NOT be substituted, as the effects of the neurotoxins produced by these other strains are not as predictable and may lead to a greater incidence of life-threatening complications. Likewise, this procedure should not, in our opinion, be performed without image guidance, due to the risk of injection of non-target structures such as vessels or facial nerve branches, with the consequently elevated risk of life-threatening complications.
Sterile US probe cover
Ultrasound with high-frequency transducer
Skin preparation liquids
22- to 27-gauge, 1.5-inch needles × number of glands to be injected
Botox®: appropriate volumes and units drawn up into 1 ml syringes prior to procedure
Patient preparation
Due to the nature of most candidates for this procedure, they are already at risk for aspiration and do not have normal airway protective mechanisms. Therefore it does not make sense to perform this procedure with sedation, which may further compromise pulmonary integrity and function. Children tolerate this procedure well without local anesthetic or adjunct medications. However, in some practices children may undergo general anesthesia. All four glands can be safely and accurately treated in about 15 to 30 minutes.
Dosage
There are different protocols that one can use to select a starting dose of Botox®. One can calculate the dose at 0.5 U/kg/gland or use a weight-based dose of15 U/gland for children <15 kg, 20 U/gland at 15 to 25 kg, and 25 U/gland >25 kg. We have found that the sensitivity of children to Botox® may vary and the interventionalist needs to be attentive to the effect and the child’s comfort. If injection produces dry mouth, the dose should be decreased at the next treatment (usually by 5 U), and if there is still drooling or a short interval between return of symptoms, the dose can be increased so that the desired effect is titrated. The length of time Botox® has an effect varies between three and eight months in most children.
The other decision to consider is whether to inject only the submandibular glands or both submandibular and parotid glands. Some interventionalists will start with the submandibular glands, since they are responsible for the basal saliva production, and add the parotids if needed. Other practitioners inject all four at the initial visit. There is no data to tell us the best approach at this time.
Standard technique
Each gland has its own characteristic appearance, and the gland must be positively localized with US before access (Table 7.10).
General anesthesia as needed
Antiseptic preparation
Determine dosage of Botox® per gland and prepare and label 1 ml syringes
Ultrasound evaluation and route planning
Ultrasound-guided needle placement within salivary gland with injection of Botox®
Parotid gland
The parotid gland is wedge shaped, found in the pre-auricular space posterior to the angle of the mandible, with the base of the wedge oriented superiorly, tapering to a point inferiorly. The parotid is also divided into deep and superficial lamina by a false capsule of investing cervical fascia and the risorius muscle. Since facial nerve branches and larger vessels run deep to or through the deep lamina, the injection needle should not go deeper than the superficial lamina. To reduce the chance of facial nerve paralysis, a nerve stimulator may be used to attempt nerve activation prior to Botox® injection. If no nerve activity is observed, Botox® is injected using a standard 22- to 27-gauge, 1.5-inch needle attached to a 1 ml syringe containing botulinum toxin A mixture (usually in 0.5 to 1 ml total volume). The needle is advanced percutaneously under real-time US guidance in the long plane of the linear transducer, entering the substance of the gland and traversing its length, staying above the superficial lamina. In this way, the location of the injection in a desirable distribution is assured before the injection is begun. The syringe is then emptied as the needle is withdrawn, “lacing” the length of the gland (Figure 7.5).
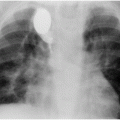
Nine-year-old male with cerebral palsy and hypersalivation with recurrent aspiration pneumonia.
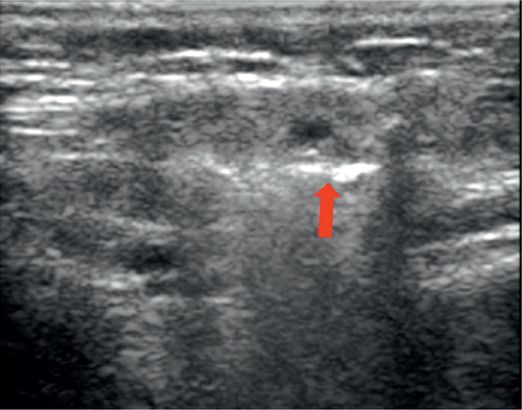
Ultrasound-guided needle positioning (arrow) in parotid gland.
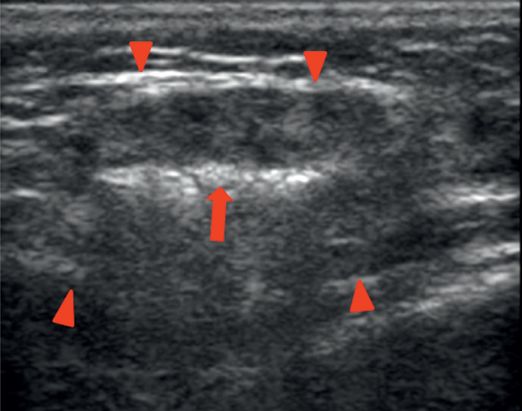
“Lacing” hyperechoic linearity (arrow) of gland (arrowheads) with Botox®.
Submandibular gland
The submandibular gland is somewhat cuboid to bilobed, located just medial to the inferior margin of the mandible, and just anterior to the inferior aspect of the parotid gland. The deep portion of the gland is the larger portion, but also contains the gland’s Wharton’s duct, the glandular branch of the facial artery, and the nerve branches leading to the submandibular ganglion. The superficial portion of the gland, although the smaller part, is more accessible and is the safer target for this procedure. Using aseptic technique and US guidance a 22- to 27-gauge, 1.5-inch needle attached to a 1 ml syringe containing botulinum toxin A mixture (usually in 0.5 to 1 ml total volume) is advanced into the superficial part of the gland, and the mixture is laced across the gland as described above.
Technical variations
Some operators prefer to inject the dose into each gland as a “depot” bolus rather than “lacing” the gland as described above. There is no evidence that either technique yields different results or complications.
Postprocedure and follow-up care
No specific postprocedural care is usually needed. A dressing or bandage is unnecessary. If anesthesia was given the child is recovered. If not, the patient is discharged to home. The caregiver should be instructed to call or activate emergency medical services immediately if the patient develops difficulty breathing, fever >38°C, difficulty swallowing, facial weakness, or changes in speech in the days following the procedure.
There is usually a lag of approximately three to seven days until the therapeutic effect of the treatment is achieved. This effect persists for several weeks and the patient normally returns to baseline salivation within three to eight months after treatment. In our experience, most patients see decreasing effectiveness around three to four months after injection. The reduction in frequency of aspiration-related complications may last for a year or more. The procedure should not be repeated more often than every two to three months. The interval between treatments varies and should be tailored to the child’s needs. There are unsubstantiated reports of a cumulative effect of serial treatments leading either to gland atrophy and reduced salivary output or to attenuation of the response with increased salivary output.
Complications
It is uncommon to observe complications following US-guided chemodenervation of the salivary glands. Most reported adverse effects involve injections without image guidance or after use of other Botulinum strains. The most severe potential complications of botulinum toxin A administration result from non-target distribution, especially when inadvertent intravascular injection occurs. This can cause severe muscle weakness in areas remote from the injection, most dangerously involving muscles of respiration. There can also be problems with vision, swallowing, heart rate, or bladder control. Local effects are also possible, including facial palsy, impairment of chewing or swallowing, as well as bruising, bleeding, swelling, or pain at the site of injection. Transient dysphagia can occur, perhaps due to changes in the viscosity of saliva. Transient xerostomia has also been reported in some patients.
Future advances
In most cases, the underlying neurologic dysfunction that results in excessive drooling and associated aspiration-related and other complications is lifelong and irreversible. The chemodenervation procedure with botulinum toxin A is temporary and self-limited. The more permanent solution currently available, gland resection, carries a high complication rate. Our experience with partial gland ablation for ranula, described below, raises the question whether this technique could be used, with appropriate neurophysiologic monitoring, to achieve a graded gland ablation, titrated to effect over a series of procedures, with the likelihood that the reduction in salivary output would last five years or more rather than five months or less.
Conclusions
Complications related to excessive drooling can be life-threatening, including aspiration-related reactive airways disease and recurrent aspiration pneumonia. These complications may result in numerous hospitalizations. In patients at highest risk, this may require more than 15 intensive care unit days per year, and may add over $100,000 per patient per year to their health care costs as well as significantly disrupting the lives of caregivers and families. Conventional pharmacologic therapy is often unsatisfactory, with undesirable side effects and uncertain benefit in many patients. Surgical gland excision is technically difficult, is not titratable or reversible, and has a high complication rate. Chemodenervation of the salivary glands with botulinum toxin A, under US guidance, is a safe and effective alternative that can be performed on an outpatient basis. It can significantly improve the lives of patients and caregivers alike, and can provide significant reduction in health care costs in patients at risk.
Partial gland ablation and sclerotherapy of ranula
Introduction
Disruption of the delicate ducts draining the salivary glands results in extravasation of serous and mucinous fluids into the periglandular soft tissues. As these fluids accumulate, they cause a local swelling, usually confined to the floor of the mouth, constituting a simple ranula. The simple sublingual ranula is usually treated by transoral surgical excision of the ipsilateral gland and evacuation of the ranula. Surgical alternatives include marsupialization with or without packing, micro-marsupialization, laser cyst, and partial gland ablation. When the accumulation dissects through the mylohyoid muscle, beyond the oral cavity, it becomes a “plunging” ranula, and may present as a swelling in the neck, submandibular, or pre-auricular region. If treated surgically, ranulas located in the neck generally require a transcervical approach with division of the mylohyoid muscle and dissection up to the floor of the mouth. While most ranulas arise from the sublingual glands, they may be related to any salivary gland, major or minor.
Swelling in the neck of a child may be solid or cystic. Since a plunging ranula should not mimic a solid mass, this can be virtually eliminated on the basis of imaging. This leaves infections (necrotic lymphadenopathy, abscess), and cystic lesions. The latter might commonly include branchial cleft cyst, thyroglossal duct cyst, lymphatic malformation, dermoid cyst, and ranula. Dermoid cysts are thin-walled cysts that contain fatty masses and may contain other differentiated ectodermal structures such as hair or nails. Branchial cleft cysts may extend any distance from the skin, usually along the margin of the sternocleidomastoid muscle, as deep as the tonsillar pillars, external auditory canal, or piriform sinus. A thyroglossal duct cyst is characteristically a midline structure between the isthmus of the thyroid and the hyoid bone. Cervicofacial lymphatic malformations are cystic lesions resulting from malformation of normal lymphatics. Most of these may enlarge with upper respiratory infections. They may also enlarge with internal hemorrhage, which may complicate the imaging appearance of these cystic structures. Solid and cystic cervicofacial masses in a child could also represent less common entities, such as teratoma or very rare malignancies such as metastatic squamous cell carcinoma or papillary cystadenocarcinoma, but are seldom confused with a ranula. Still, a fine needle aspirate from a complex fluid collection or a core biopsy of a complex cystic and solid tumor will usually provide adequate diagnostic direction to the clinical team.
An anechoic cystic neck mass can successfully be treated in almost all circumstances via drainage and sclerotherapy using the same techniques described for treatment of lymphatic malformations in Chapter 8. However, if there is any turbidity or granularity in the fluid by US, or if the diagnosis of ranula is seriously considered, then a sample of the fluid should be sent for salivary amylase analysis. Caution should be used if undifferentiated amylase analysis is substituted, as there is a possibility of confusing a foregut duplication cyst, which is a poor candidate for sclerotherapy, with a ranula, where sclerotherapy may contribute to a successful treatment plan.
Indications
Classically, a hypoechoic cystic (or pseudocystic) structure extending from the floor of the mouth, adjacent to a salivary gland, with a turbid or granular appearance on US and which contains a significant level of salivary amylase (>100 mg/dl) is a ranula. (Non-fractionated amylase may be found in other cystic foregut structures; Figure 7.6). As described above, simple ranulas are most easily and effectively treated by surgical excision of the gland and evacuation of the ranula. All other ranulas are candidates for partial ablation of the parent gland and sclerotherapy and drainage of the ranula collection. It is important to understand that testing for salivary amylase will usually require submission of the sample to an outside lab, and that a final result may not be available for one to two weeks.
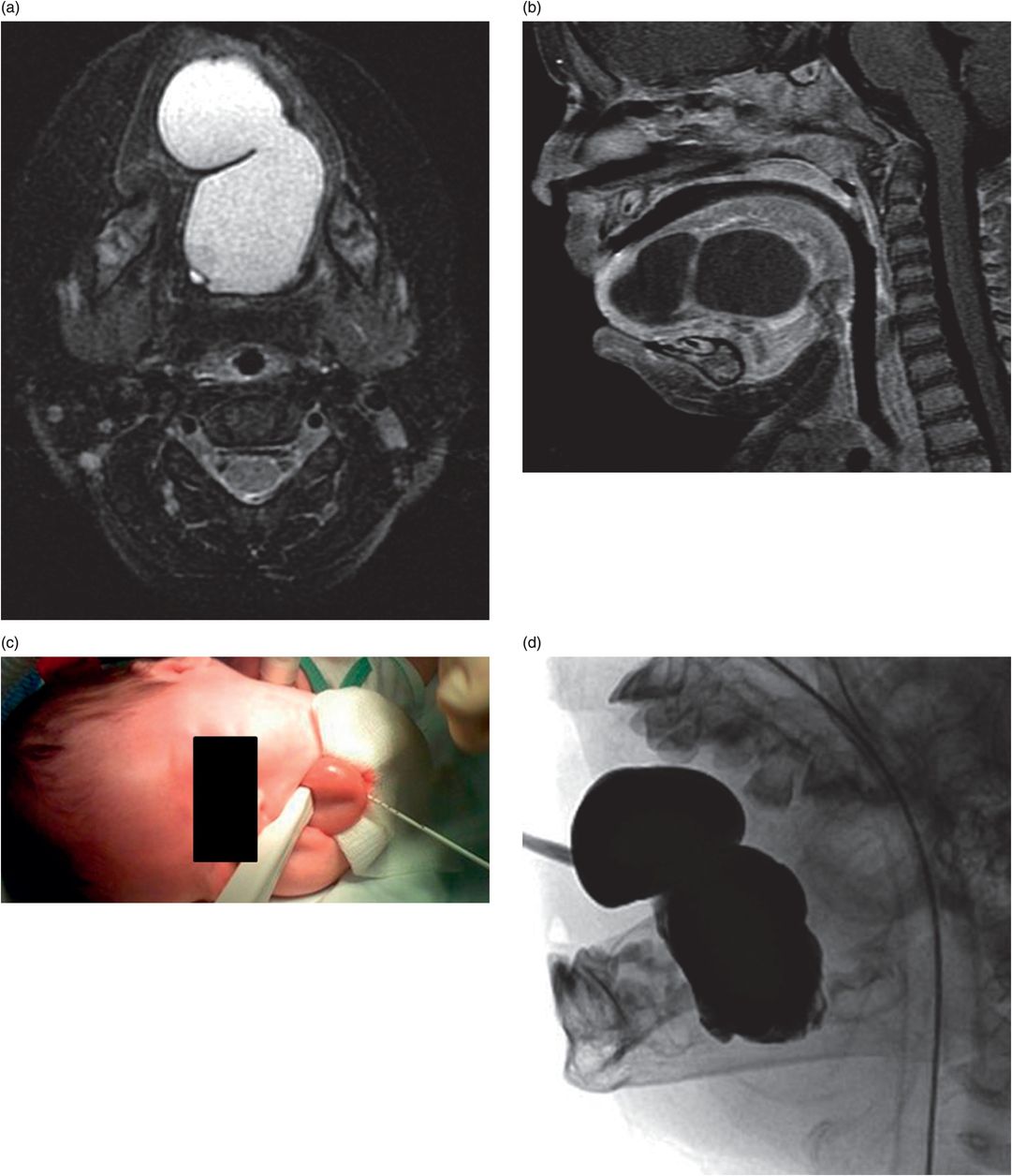
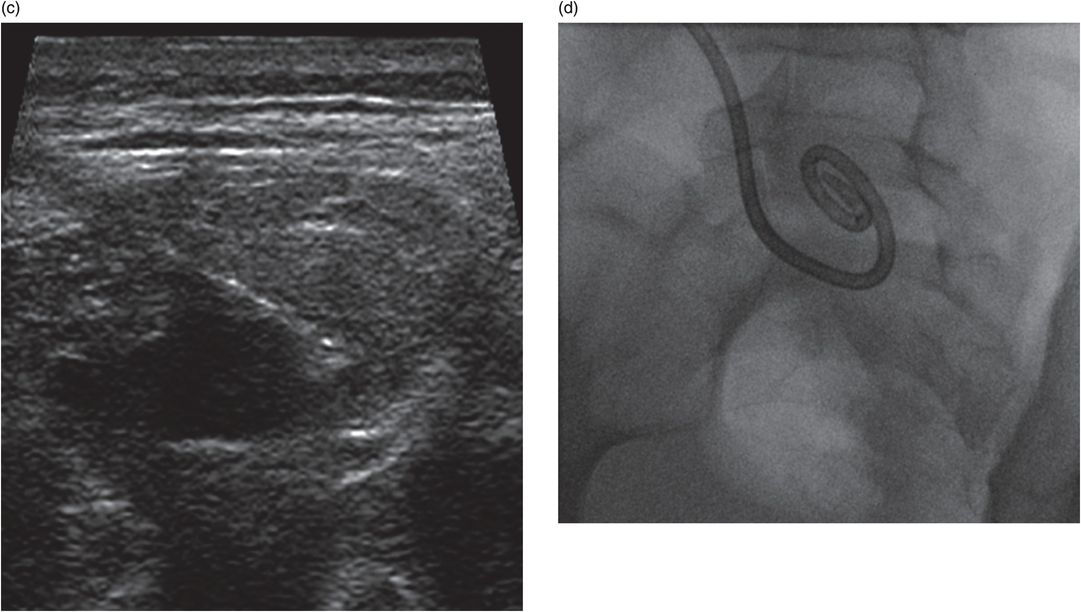
Infant presenting with painless intralingual mass and positive unfractionated amylase from aspirated fluid. (a,b) Axial T2 and sagittal T1 images show a macrolobulated cystic lesion of the tongue and floor of mouth. (c) Transglottic US-guided drain placement. (d) Cyst opacification prior to sclerotherapy and attempted partial sublingual gland ablation. After multiple interventions, salivary amylase was tested and was substantial <100 mg/dl. Pathology after surgical excision showed an intralingual foregut duplication cyst.
There is no absolute contraindication to this approach in a properly diagnosed ranula. Relative contraindications would include inability to identify the parent gland and inability to localize and ablate the portion of the parent gland adjacent to the ranula collection without stimulating, and thus risking injury to, the facial or lingual nerve. For a suspected ranula in the midline, it is helpful to document a normal thyroid gland with appropriate imaging to avoid inadvertent ablation of an ectopic thyroid gland. In the event the skin overlying a ranula is infected, a transoral route may be considered, or the procedure can be delayed until the infection is cleared.
Technique
Equipment
The equipment and supplies for sclerotherapy of the ranula collection are similar to that described in the “Sclerotherapy” section in Chapter 8. This includes a 5- to 8.5-French percutaneous drainage catheter, depending upon the location and the size of the collection, suture (e.g., 3–0 Vicryl or 3–0 silk), 1% buffered lidocaine, 3% sodium tetradecyl sulfate, and dehydrated alcohol (Table 7.11). For partial gland ablation, supplies include a 3 ml syringe containing dehydrated alcohol connected either directly or via a short connecting tube to a 27-gauge, 1.5-inch needle. For this procedure, consideration should be given to intraprocedural neurophysiologic testing for proximity to motor branches with a nerve stimulator connected to the 27-gauge needle, especially if planning to treat the parotid gland. Consultation with a neurologist or anesthesiologist may be helpful for this aspect of the procedure.
Sterile US probe cover
High-frequency US transducer with small footprint
Skin preparation liquids
Micropuncture or Yeuh sheathed needle
5- to 7-French pigtail drain
27-gauge, 1.5-inch needle
Connecting tube
Syringes, three-way stopcock
1% lidocaine
Dehydrated alcohol
Sodium tetradecyl sulfate
Patient preparation
Preprocedure imaging routinely includes US, and may be supplemented by MRI in cases where the diagnosis or the gland of origin is uncertain. The procedure may be performed under general anesthesia or with procedural sedation. The anesthesia team should be advised that treatment of a lesion in the floor of the mouth may be accompanied by significant swelling that could potentially compromise the airway. If this is a concern, provision for a prolonged period of postprocedure monitoring, or even provisional admission to the intensive care unit, and delay of extubation until the airway is cleared, may be necessary.
Standard technique
Sclerotherapy
The ranula is accessed with an angiocatheter or single-wall needle sized to admit the planned guide wire, under real-time US guidance using a linear transducer with good near-field imaging capability. The pseudocyst fluid is aspirated and the volume noted. A sample is saved for laboratory analysis. A pigtail drainage catheter (5- to 6.3-French with 1 cm pigtail) is inserted into the collection using a standard technique over a 0.018-inch angled Glidewire® or stiff guide wire for 5-French drains or a 0.035-inch angled Glidewire® for 6.3-French drains.
The size and nature of the ranula is evaluated with non-ionic contrast (e.g., Optiray™ 300) injected through the drainage catheter in a volume similar to the aspirated volume. The cystic space is then anesthetized with a half volume (not exceeding 1 ml/kg) of 1% lidocaine for ten minutes, if using local anesthesia only or sedation. Next, sodium tetradecyl sulfate is instilled into the cyst (not exceeding 1 ml/kg) for three to five minutes. Finally, sclerosis is completed with a half-volume (not exceeding 1 ml/kg) of dehydrated alcohol for 15 minutes. Each agent is completely aspirated through the catheter at the end of its dwell time. At the completion of the procedure, the drainage catheter is left to bulb suction drainage. Successful end of therapy is determined by inability to easily inject an appreciable volume of contrast through a 10 ml syringe connected to the drainage catheter. The drain is removed at the end of therapy.
Partial gland ablation
Successful treatment for ranula with sclerotherapy may require several sclerotherapy sessions over several months. Partial gland ablation is usually definitive and significantly reduces the time required to achieve a successful outcome (Table 7.12). However, it makes sense to delay progression to partial gland ablation until the diagnosis is confirmed with salivary amylase determination. When partial gland ablation is performed, the gland of origin is determined by intraprocedural US as the gland adjacent to or nearest in proximity to the cystic space prior to drainage. If there is uncertainty, preprocedural MRI of the region can help to clarify relevant anatomic relationships, especially when the normal architecture of the gland is significantly altered by the ranula collection (Figure 7.7).
General anesthesia
Ultrasound examination for route planning
Preparation and draping
Ultrasound-guided placement of a drainage catheter
Intralesional contrast injection to determine pathologic anatomy
Sclerotherapy of ranula
Connect to bulb suction device
Partial gland ablation with dehydrated alcohol
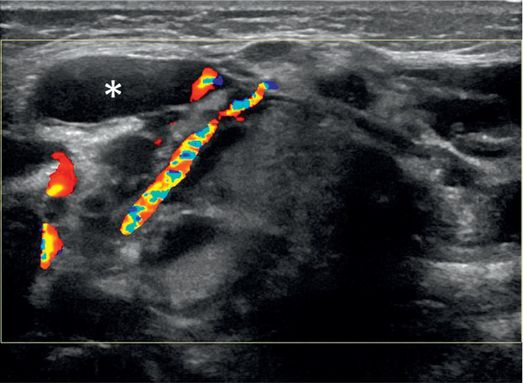
Ultrasound of the submandibular region shows mixed solid and cystic components with normal vessels. A sample of the granular, turbid fluid (asterisk) showed an abnormal level of salivary amylase.
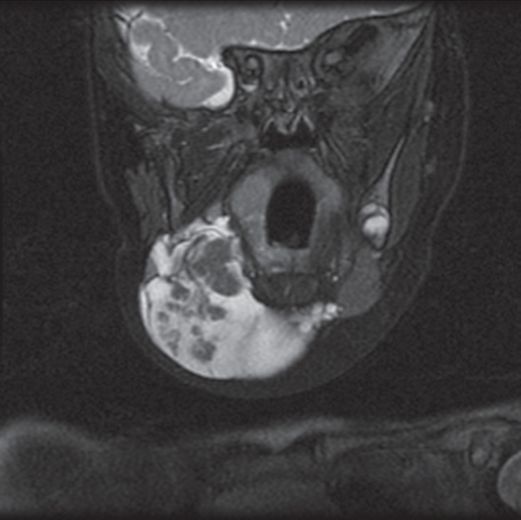
A coronal T2 fat saturation MR image shows the submandibular gland with an “exploded” appearance of the superficial lobe within the ranula fluid.
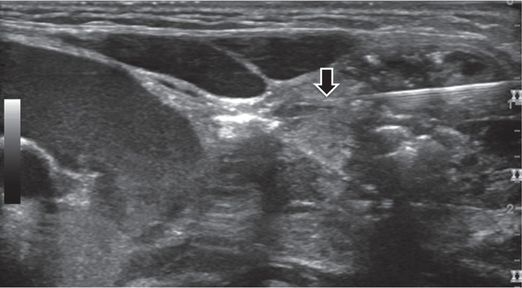
A 27-gauge, 1.5-inch needle is positioned under US guidance with its tip (arrow) within the parenchyma of one of the gland fragments adjacent to abnormal fluid.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree