Chapter 138 Etiology, Pathophysiology, and Clinical Presentation: Osteomyelitis may occur from direct or hematogenous inoculation; it may be iatrogenic, related to orthopedic implants; or it may be from secondary extension from primary septic arthritis or pyomyositis. Hematogenous osteomyelitis is preponderantly a disease of children; however, infantile and even neonatal cases are not uncommon. Bacteria are the most common inflammatory agents, but growing bones may also be invaded by other pathogens, including viruses, spirochetes, and fungi. The incidence of pediatric osteomyelitis in the United States is 1 in 5150 and has increased 2.8-fold in 20 years.1 This increased incidence is confounded, however, because of differences in access to health care and advances in imaging diagnosis. Staphylococcus aureus remains the most common causative organism of acute osteomyelitis in children. Unfortunately, community-acquired methicillin-resistant S. aureus (MRSA) strains are increasing in prevalence.2 Haemophilus influenzae osteomyelitis and septic arthritis have become less common since the availability of effective vaccination (H. influenzae type B vaccine).3 In sickle cell disease, bone complications include osteonecrosis and osteomyelitis. Osteonecrosis is approximately 50 times more frequent than osteomyelitis.4 The proposed mechanism of osteomyelitis is hematogenous, with bacteria gaining entrance to blood vessels through ischemic bowel and finding suitable culture material in foci of infarcted bone marrow. Both S. aureus and Salmonella commonly occur in sickle cell patients.2 In chronic granulomatous disease of childhood, an X-linked recessive disorder of leukocyte function, repeated infections occur in solid organs, skin, and bone. Approximately one third of patients develop osteomyelitis. Phagocytes are unable to kill catalase-positive organisms such as Staphylococcus and Aspergillus.5 Hematogenous osteomyelitis usually involves the highly vascularized metaphysis of the fastest growing bones, such as the distal femur and radius and the proximal tibia and humerus. The most common location for hematogenous osteomyelitis is about the knee (distal femur, proximal tibia).2 Pain, localized signs, fever, reduced range of motion, and reduced weightbearing are the most common initial clinical features. A history of trauma is seen in approximately 30% of cases,2 and the male-to-female ratio is approximately 1.8 : 1. Organisms lodge most frequently in the terminal capillary sinusoids of the metaphyses.6 Rarely, they may locate initially in the epiphyses related to the terminal capillary sinusoids of the metaphyseal equivalent region immediately next to the spherical growth plate (Fig. 138-1). A small abscess forms in the marrow of the metaphysis, followed by local decalcification and destruction of the adjacent bone. When focal abscesses are generated, multiple small foci of bone destruction develop and later coalesce. Inflammatory swelling increases the intraosseous pressure because of the rigid bony walls of the marrow cavity; this can force extension of the infected exudate into several sites, as indicated in Figure 138-2. The most common route is via the haversian canals of the cortex to the subperiosteal space, where a subperiosteal abscess is formed. Simultaneously, spread also occurs farther within the medullary cavity. Rupture of the periosteal abscess is responsible for extension of infection into the adjacent soft tissues. Inflammation and rapidly increased intraosseous pressure may cause thrombosis of the vascular channels. Figure 138-1 The blood supply to the metaphysis and epiphysis of a child and the arterial channels through which invading organisms enter the growing bone. Figure 138-2 Pathways of infection after hematogeneous implantation in the metaphysis and formation of a metaphyseal focus (M) of bone infection (bone abscess). The most common location for direct inoculation osteomyelitis is the foot. Plantar puncture wounds secondary to walking on broken glass, metal (nail), or vegetable matter (thorn, toothpick) may result in infectious cellulitis, plantar fasciitis, and osteomyelitis, whether the foreign body is removed or retained. The calcaneus is often involved, and Pseudomonas aeruginosa is often found related to direct inoculation, usually with a history of a puncture through a shoe.5 Imaging: Imaging guidelines for the evaluation of suspected osteomyelitis include radiographs first to exclude alternative etiologies, such as a fracture or neoplasm, as an explanation for symptoms. If radiographs are normal, ultrasound (US) is recommended if the symptoms are localized to an osteoarticular region. If US is negative, scintigraphy is the next study of choice, if symptoms are nonlocalizable.7 If symptoms can be localized, targeted magnetic resonance imaging (MRI) of the affected region should be performed both for diagnostic purposes and for planning surgical treatment.8,9 Radiography: With acute osteomyelitis, the earliest change on radiographs is soft tissue swelling; osseous changes are seldom present until the second week of disease (Fig. 138-3).10 The earliest bone changes seen on conventional images are one or more small radiolucencies, usually in the metaphyseal region, where necrosis and destruction of bone has occurred (Fig. 138-4). On serial examinations, these areas of bone destruction enlarge and become confluent. Figure 138-3 Osteomyelitis in a 6-year-old girl. Figure 138-4 Early radiographic changes of osteomyelitis in the proximal left humeral metaphysis. With continuing appropriate antibiotic therapy, periostitis is visible when the periosteum begins to produce new bone on its undersurface after the second or third week (Fig. 138-5). Osteogenic function by the periosteum suggests that infection has been at least partly locally controlled. Subsequent healing may involve remodeling of the cortical new bone and reconstitution of the underlying bone or, if damage has been extensive, it may involve an increase in the amount of periosteal reaction to form an involucrum (Fig. 138-6), a living bone sheath around the fragments of the old devitalized bone (sequestrum). Figure 138-5 Osteomyelitis in a 12-year-old boy. Scintigraphy: Early bone scans may demonstrate a “cold” metaphyseal lesion as a result of compression or occlusion of the metaphyseal vessels. In these cases, increased activity is observed toward the diaphysis, beyond the cold metaphyseal area, which subsequently becomes “hot” and merges with the adjacent increased activity. The multiphase bone scan is very sensitive and is usually positive 24 to 48 hours after the onset of symptoms.7 It can detect extension of metaphyseal osteomyelitis into the epiphysis through the growth plate (Fig. 138-7). Figure 138-7 A 4-year-old boy with fever and inability to walk or move the left lower extremity for 6 days. In the early detection of acute bone infection, radionuclide imaging is more sensitive than radiographs and can identify additional foci of disease not clinically apparent. Vascular phase images done within the first 5 minutes following injection, delayed images with pinhole collimators, and special attention to the affected area have proven of great value. Osteomyelitis appears as an area of increased tracer activity that reflects the hyperemia and bone turnover induced by the infectious process. In a study of 100 children with acute limb pain, the sensitivity and specificity of three-phase bone scans for acute osteomyelitis were 84% and 97%, respectively.11 Errors arise from simulation of infection by fracture or sickle cell disease, obscuration of osteomyelitis by septic arthritis, prior antibiotic treatment, and “cold” defects that result from ischemia. It is difficult to detect infection close to the growth plate, because both the growing physis and the nearby area of infection show increased activity. Computed Tomography: Computed tomography (CT) is of limited clinical value in acute osteomyelitis.12 It is more useful in advanced or chronic disease to help determine the quality of bone stock, including determinations of cortical destruction, involucrum, and sequestra (see Fig. 138-6). Magnetic Resonance Imaging: MRI is the optimal study to evaluate for infection and alternative etiologies for symptoms, particularly when radiographs are normal. MRI can identify early bone changes, and it delineates the anatomy and extent of marrow involvement; for this reason, it has become an important tool for imaging of suspected osseous infection. However, MRI does carry additional cost, and depending on the availability of scanner time and the need for sedation or anesthesia, delays in definitive diagnosis and treatment are possible. Imaging should aim at guiding or modifying treatment if necessary. When precontrast MRI exams are entirely normal and show no evidence to suggest osteomyelitis, routine post-gadolinium images may not be necessary.13,14 When abnormal, MRI can reveal marrow alterations and extent of disease in bone, soft tissues, or adjacent joints (Fig. 138-8).15 Early MRI findings of osteomyelitis may have a tumefactive appearance and may be paradoxically hypointense on fluid-sensitive sequences (e-Fig. 138-9). Over time, the lesion may remain masslike and demonstrates the expected, more homogeneous hyperintense signal on fluid-sensitive sequences, indicative of its inflammatory nature (Fig. 138-10). Eventually, periostitis and adjacent soft tissue involvement may be seen in the early phase of osteomyelitis (e-Fig. 138-11). Subperiosteal abscess formation may be seen by sonography (Fig. 138-12) or MRI (Fig. 138-13), preceding radiographic bony changes. A salt-and-pepper appearance to the marrow may be seen in the late acute phase of osteomyelitis and is presumed to represent small areas of noncoalescent microabscess formation and early bone destruction (Fig. 138-14). Figure 138-8 Distal tibial osteomyelitis and tibiotalar septic arthritis in an 11-year-old boy. Figure 138-10 Child with biopsy-confirmed osteomyelitis of the distal femur. Figure 138-12 Acute osteomyelitis of the distal fibula in a 12-year-old boy. Figure 138-13 Acute osteomyelitis of the distal femur in a 5-year-old boy. Figure 138-14 Femoral osteomyelitis in a 13-year-old girl. e-Figure 138-9 Biopsy-confirmed osteomyelitis in a 13-year-old boy. e-Figure 138-11 A 12-year-old girl with proximal humeral osteomyelitis. Subacute or chronic osteomyelitis may develop as a result of partial host response to contain the infection. Distinguishing between subacute and chronic osteomyelitis is arbitrary.16 The initial purulent exudate is replaced by granulation tissue, and the clinical manifestations are mild and consist mainly of local pain. A Brodie abscess may then develop, typically in the metaphysis and less commonly in the epiphysis, because the growth plate is only a partial barrier against the spread of infection. A Brodie abscess is characterized radiographically by a central or eccentric round or oval radiolucency.17 The cavity may contain a small, dense sequestrum. On MRI, lesions have a characteristic layered appearance with a high-signal periphery as a result of edema (penumbra sign)18 and a double-line sign (rim sign), which on fluid-sensitive sequences is delineated as a low-signal outer rim because of sclerosis; an inner rind of intermediate signal because of granulation tissue; and a central, hyperintense region related to abscess (Fig. 138-15).19 With contrast-enhanced imaging, the inner granulation layer will show enhancement around the nonenhancing central abscess. Figure 138-15 Brodie abscess in a 3-year-old boy. Adjacent soft tissue swelling and edema and periosteal new bone formation may be present. In spite of occasional growth plate involvement, the incidence of premature growth plate fusion after subacute osteomyelitis is rare.20 Cortical and trabecular bone sclerosis, cavities, involucra, and sequestra are characteristic of advanced osteomyelitis.21 The affected bone is thickened, and its outline may be wavy, with or without periosteal cloaking of new bone. An involucrum of reactive, viable bone may cloak an area of infection (Fig. 138-16). The involucrum may be perforated by a cloaca, which is a tract or communication between bone and the surrounding soft tissues (e-Fig. 138-17). If the cloaca extends to the skin surface, it is termed a sinus tract (see Fig. 138-16).22 The necrotic, devitalized bone of a sequestrum is surrounded by inflammatory granulation tissue and may be located within a bone abscess cavity. The dead bone of a sequestrum is relatively sclerotic. Sequestra may be demonstrated on radiography, CT, or MRI, but it is best seen with CT (see Figs. 138-6 and 138-16). Sequestration is now relatively rare owing to earlier diagnosis, largely because of advances in imaging, and more effective antibiotic therapies. Figure 138-16 Chronic osteomyelitis of the distal radius in a 13-year-old girl. e-Figure 138-17 Chronic osteomyelitis and cloaca in a 17-year-old boy. Radiographs will suggest the diagnosis of subacute or chronic osteomyelitis and may be used to follow up for gross changes. Although CT is of limited clinical value in acute osteomyelitis, it is very useful in imaging chronic disease to evaluate bone stock quality and to detect cortical metaphyseal tracts and channels, sequestra, and bone destruction. MRI will detect any reactivation or persistence of infection by showing focal active disease in the bone marrow and also showing juxtacortical soft tissue hyperemia and edema. Serial MRI after an established diagnosis of osteomyelitis has been made, and after medical or surgical treatment, has limited utility.23 Treatment: Although the mortality and morbidity of bone infection have decreased significantly, permanent sequelae do occur, largely as a result of delay in diagnosis or inadequate treatment with complications related to generalized bacterial sepsis.24 Complications of bone infection include pathologic fracture through regions of bone destruction,25 venous thrombosis,26 and adjacent infectious arthritis and destruction of joints (Fig. 138-18). Figure 138-18 A 14-year-old boy with a remote history of distal femoral osteomyelitis and septic arthritis. Identification of a bacterial pathogen for underlying osteomyelitis ranges from 9% to 22% by blood culture and 40% to 50% by bone or joint aspiration.24
Musculoskeletal Infections
Overview of Musculoskeletal Infections
Acute Pyogenic Osteomyelitis
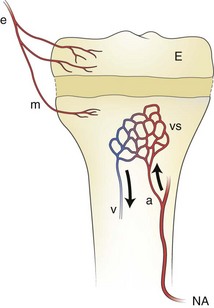
An epiphyseal artery (e) supplies the epiphysis (E) and may branch to give (minor) metaphyseal vessels (m). The major blood supply of the metaphysis comes from the nutrient artery. a, arteriole; NA, nutrient artery; v. venule; vs, venous sinuses in the metaphysis.
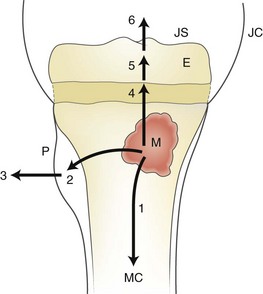
1, spread to the medullary canal (MC); 2, formation of a subperiosteal abscess; 3, penetration of the periosteum (P) and spread to the adjacent soft tissues; 4, 5, and 6, spread across the growth plate to the epiphysis (E), and eventually to the joint space (JS). JC, joint capsule.
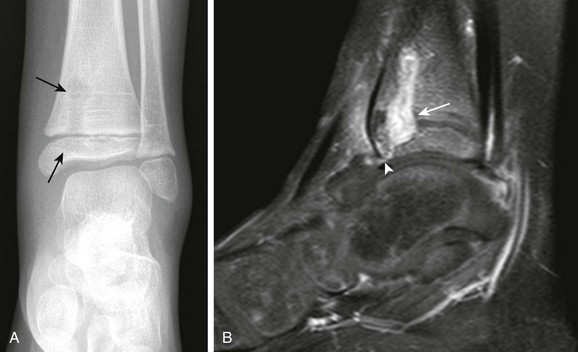
A, Frontal radiograph demonstrates a lytic lesion in the distal tibia metaphysis extending into the epiphysis (arrows). B, T1-weighted fat-saturated post-gadolinium sagittal view demonstrates a thick, rim-enhancing lesion with a small amount of nonenhancing fluid consistent with early abscess formation with epiphyseal extension (arrow) and a small cloaca (arrowhead) extending to the tibiotalar joint.
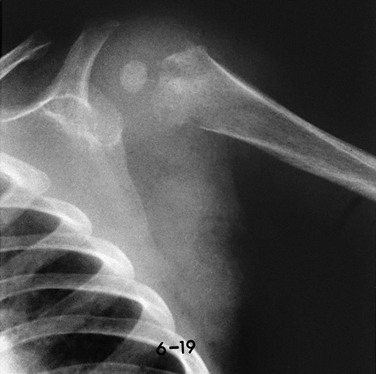
This 9-month-old boy had fever and local signs and symptoms for 12 days. Metaphyseal areas of bone destruction are visualized as irregular, ill-defined lucencies.
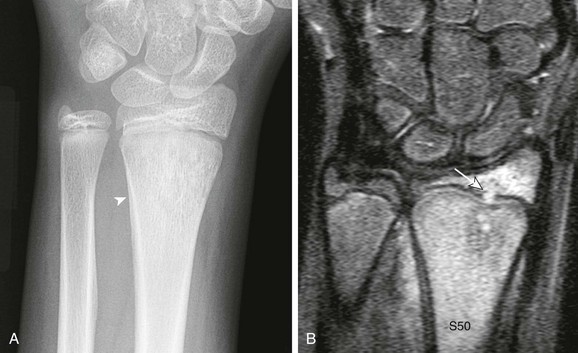
A, Frontal radiograph demonstrates distal radial moth-eaten bone destruction with periostitis (arrowhead). B, Short tau inversion recovery coronal image demonstrates diffuse marrow edema with transphyseal extension (arrow) into the epiphysis.
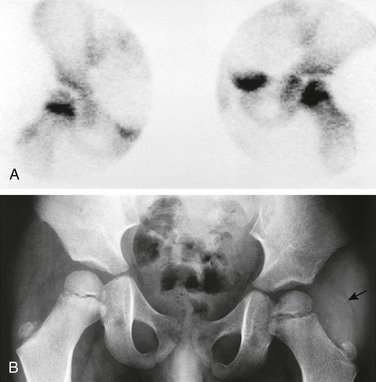
A, Bone scan shows increased uptake in the femoral neck and head on the left side, likely resulting from extension of an initial metaphyseal focus of infection into the epiphysis. The right hip shows normal increased uptake in the region of the growth plate. B, Radiograph of the hips 2 weeks after the beginning of symptoms shows osteopenia on the left side, ill-defined medial metaphyseal and epiphyseal bone lucencies on each side of the growth plate, and indirect evidence of left hip joint effusion. Fat line (arrow) is displaced laterally by the joint fluid. Adjacent deep soft tissues also appear swollen and edematous compared with the normal right side.
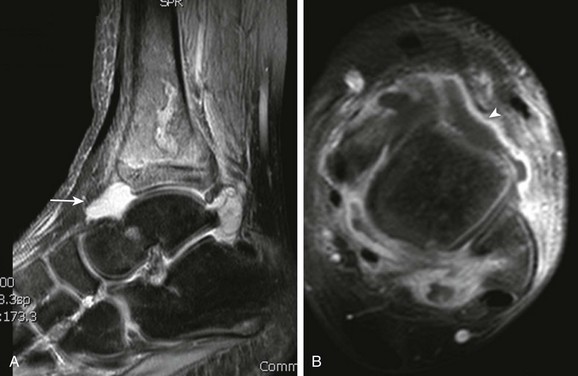
A, T2-weighted fat-saturated sagittal magnetic resonance image demonstrates diffuse marrow edema that includes transphyseal extension to the epiphysis and juxtacortical soft tissue edema. A large joint effusion is present (arrow) with T1-weighted fat-saturated post-gadolinium axial images (B) that demonstrate thick synovial enhancement (arrowhead).
A, T1-weighted coronal magnetic resonance imaging (MRI) demonstrates tumefactive marrow replacement in the distal femur. B, Short tau inversion recovery coronal MRI demonstrates diffuse homogeneous hyperintensity in the same distribution with early physeal extension of infection (arrow).
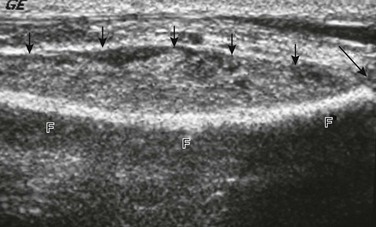
Ultrasound shows a large subperiosteal abscess (small arrows) and distal fibular growth plate (long arrow). F, fibula.
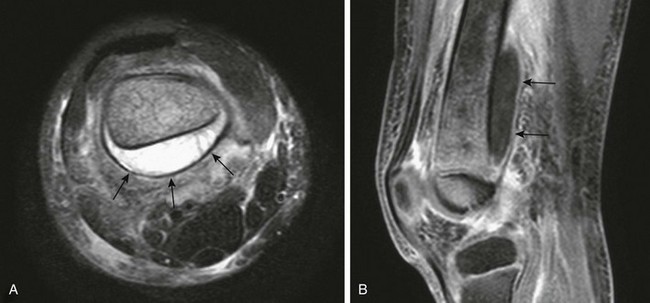
A, T2-weighted fat-saturated axial magnetic resonance imaging (MRI) shows a large subperiosteal abscess (arrows) at the posterior aspect of the femur. Increased signal is seen within the bone, and there is adjacent soft tissue edema. B, T1-weighted fat-saturated post-gadolinium sagittal MRI shows the longitudinal extent of the subperiosteal abscess with enhancing wall (arrows).
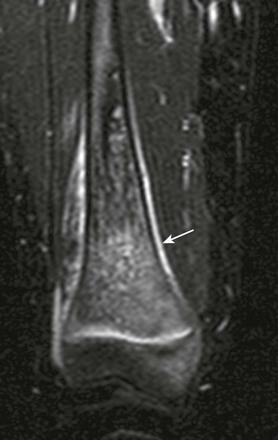
Short tau inversion recovery coronal magnetic resonance image demonstrates a salt-and-pepper appearance to marrow edema and periosteal reaction (arrow).
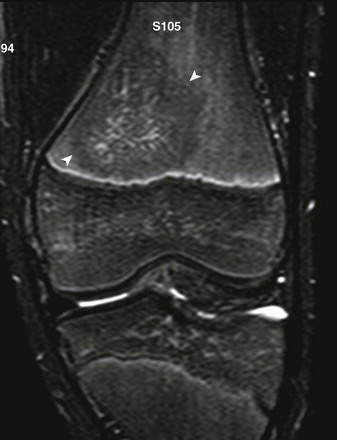
short tau inversion recovery coronal sequence demonstrates round masslike hypointensity in the distal femur metadiaphysis (arrowheads).
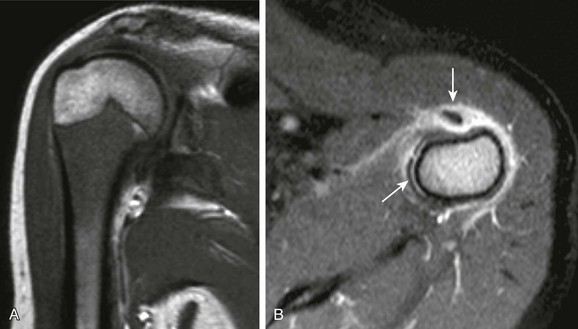
A, T1-weighted coronal magnetic resonance imaging (MRI) demonstrates diffuse masslike marrow replacement in the proximal humerus. B, T1-weighted fat-saturated post-gadolinium axial MRI demonstrates periostitis and juxtacortical soft tissue edema. Note tenosynovitis of the long head of the biceps tendon (arrow).
Subacute and Chronic Osteomyelitis
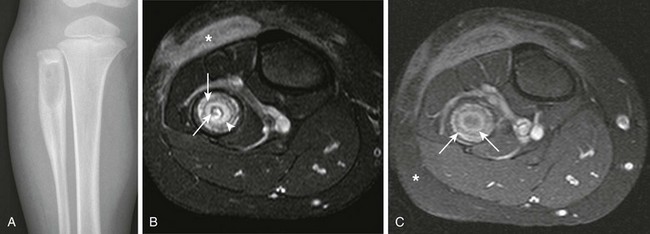
A, Radiograph demonstrates a lytic lesion in the proximal fibula with laminated thick periostitis. T2-weighted fat-saturated axial (B) and T1-weighted fat-saturated post-gadolinium axial (C) magnetic resonance images demonstrate a Brodie abscess with sclerotic outer rim (asterisk) and inner granulation tissue with enhancement (arrowhead). Note the nonenhancing central abscess, which contains a small sequestrum (arrows) that is only seen on the T2-weighted fat-saturated sequence.
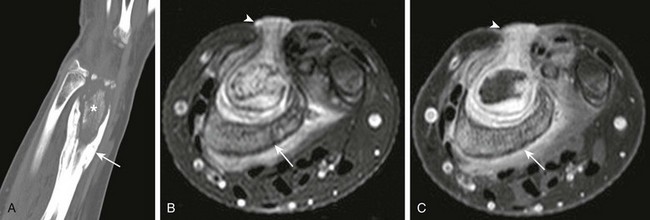
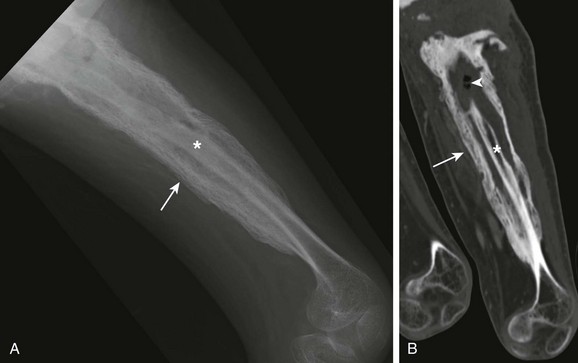
A, Computed tomographic coronal reformat shows involucrum (arrow) surrounding sequestrum (asterisk). T2-weighted fat-saturated axial (B) and T1-weighted fat-saturated axial (C) post-gadolinium magnetic resonance image shows involucrum (arrow) that surrounds the nonenhancing sequestrum. Note granulation along the sinus tract that extends from the radial metaphysis sequestrum and abscess to the skin surface (arrowhead).
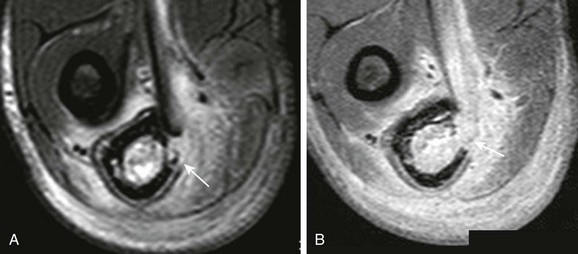
T2-weighted fat-saturated axial (A) and post-gadolinium T1-weighted fat-saturated axial (B) magnetic resonance image demonstrate cortical destruction with communication (arrow in A) between marrow and adjacent soft tissues (cloaca).
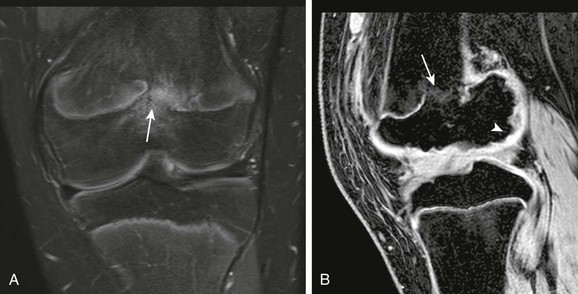
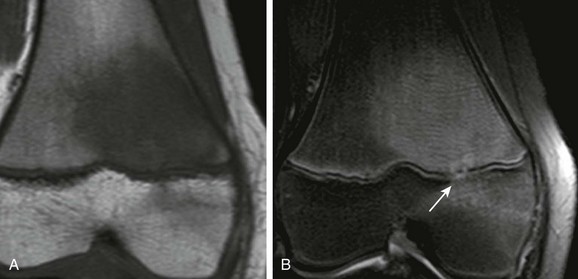
Physeal bar (arrows) and femoral condylar epiphyseal irregularity (arrowhead) are seen on T2-weighted fat-saturated coronal (A) and gradient recalled echo sagittal (B) magnetic resonance image.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree