Musculoskeletal Perfusion Imaging
George R. Matcuk Jr, MD
▪ Introduction
Musculoskeletal disorders contribute greatly to health care system costs because of their high prevalence and impact on disability, with arthritis and back pain alone affecting over 100 million individuals and costing more than $200 billion per year.1 New advances in minimally invasive surgical techniques, image-guided interventions, and other therapeutic options have spurred interest in new methods of noninvasive imaging methods capable of diagnosing disease at its earliest stages. Although they have yet to be implemented in routine clinical practice, perfusion imaging techniques have been investigated and show promise for musculoskeletal applications, including for the evaluation of normal versus abnormal bone marrow and muscle; assessment of avascular necrosis, synovitis, and chondrosis; and evaluation of musculoskeletal tumors, including recurrence and therapy response.
▪ Techniques
Dynamic contrast-enhanced (DCE) techniques can be applied to the musculoskeletal system using magnetic resonance (MR), computed tomography (CT), or ultrasound (US) in a similar manner to other areas of the body, allowing assessment of perfusion and vascularity, which will only be briefly reviewed here.
MR perfusion imaging after the intravenous administration of gadolinium-based contrast agents is the most commonly used technique and is made possible by the development of ultrafast T1-weighted gradient echo-based pulse sequences with fat saturation, such as the fast low angle shot (FLASH) sequence.2,3,4 and 5 MR has the advantage of high contrast resolution and can be done at the time of routine clinical imaging, where MR is the gold standard for imaging of most musculoskeletal disorders. An MR angiography sequence with sparse k-space sampling has been successfully implemented as a DCE-MRI technique, which eases adding this technique as part of a routine clinical MRI tumor protocol.6
Dynamic CT following the intravenous administration of iodinated contrast agents can likewise be used for musculoskeletal perfusion imaging, with the potential for higher resolution imaging and easier assessment of greater three-dimensional volumes of tissue, but at the cost of radiation dose to the patient.7 Dynamic CT may be the modality of choice for musculoskeletal perfusion imaging when the lesion is small and involves cortical bone, susceptibility artifacts limit MR, or contraindications to MR, such as a pacemaker exists.
Contrast-enhanced ultrasound (CEUS) utilizing microbubble contrast agents can also be used to assess perfusion, offering realtime dynamic imaging, which can be useful for visualizing very early or late enhancement patterns that may be missed with CT or MR and the additional ability to perform multiple repeat injections and observations as part of a single examination without radiation and contrast-related nephrotoxicity. However, the microbubble fragility and Doppler-related artifacts have limited their clinical usage. Additionally, the inability of ultrasound to penetrate bone limits the musculoskeletal applications.8
For superficial structures such as the hand and wrist, optical perfusion imaging techniques are also possible following intravenous administration of fluorescent compounds such as indocyanine green (ICG).9
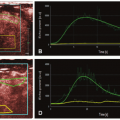
Data obtained from these DCE techniques regardless of modality used can be analyzed using both qualitative and quantitative methods. Regions of interest (ROI) chosen within a lesion can be compared to ROIs of normal structures such as uninvolved muscle or bone marrow. Visual analysis of shapes or patterns of time-intensity curves (TIC) can be used to qualitatively characterize perfusion as one of seven described types (Fig. 33-1).10 Further assessment of the TIC can include measurements of the maximum enhancement (ME), rate of early enhancement (REE), area under the curve (AUC), time to peak (TTP) enhancement, and initial slope of increase, although these values are sensitive to variations between acquisition protocols and sequence parameters. Quantitative parameters of vascular permeability (ktrans) can also be extracted from these data.
▪ Bone Marrow
Characterization of bone marrow using perfusion imaging techniques is one of the most common musculoskeletal applications and is an area of active investigation and the subject of numerous recent publications.11
Normal Marrow and Osteoporosis
Hematopoietic (red) marrow is more vascular than fatty (yellow) marrow, and therefore, younger patients (with a high percentage of red marrow within their vertebra) demonstrate greater perfusion than older patients.12,13 and 14 Women demonstrate a higher marrow perfusion rate than men younger than 50 years, but then show
a more marked decrease than men older than 50 years.12 These differences related to age and gender are likely related to associated decreases in bone mineral density and increased fat fraction, with osteopenic bone demonstrating decreased marrow perfusion relative to bone with normal density and further reduction in mean enhancement slope of osteoporotic bone on DCE-MR.15,16,17 and 18 There are also small but significant differences in marrow perfusion between vertebral levels, with decreased perfusion from upper to lower lumbar levels, possibly related to increased stresses on lower levels.19 Hypertension may also influence marrow perfusion, with decreased femoral head and proximal shaft perfusion demonstrated in hypertensive compared to normotensive rats.20 However, differences in perfusion parameters in bone marrow based on age and fat fraction are small, there is wide variation between individuals, and they are unlikely to confound changes related to malignancy or treatment.21
a more marked decrease than men older than 50 years.12 These differences related to age and gender are likely related to associated decreases in bone mineral density and increased fat fraction, with osteopenic bone demonstrating decreased marrow perfusion relative to bone with normal density and further reduction in mean enhancement slope of osteoporotic bone on DCE-MR.15,16,17 and 18 There are also small but significant differences in marrow perfusion between vertebral levels, with decreased perfusion from upper to lower lumbar levels, possibly related to increased stresses on lower levels.19 Hypertension may also influence marrow perfusion, with decreased femoral head and proximal shaft perfusion demonstrated in hypertensive compared to normotensive rats.20 However, differences in perfusion parameters in bone marrow based on age and fat fraction are small, there is wide variation between individuals, and they are unlikely to confound changes related to malignancy or treatment.21
Vertebral Compression Fractures
For acute osteoporotic vertebral compression fractures, perfusion parameters including plasma flow (PF), plasma volume (PV), extraction flow (EF), and mean transit time (MTT) are increased relative to healthy vertebral bone marrow.17,22 The increased perfusion in fractures is thought to be related to reparative neovascularization.17 Perfusion levels of normal vertebra are not significantly changed in patients with compression fractures at other levels.12
Extensive research efforts have centered on using imaging to differentiate benign (osteoporotic) versus malignant vertebral compression fractures.23,24,25,26,27,28,29,30,31,32,33 and 34 Signal intensities and morphologic features on routine MR pulse sequences alone do not offer a high degree of specificity in differentiating between these entities. Contrastenhancement and diffusion-weighted imaging (DWI) increase sensitivity and specificity, with positive and negative predictive values (NPVs) of 90%.28 MR perfusion imaging techniques have also shown promise as an additional tool, with spots of high plasma flow demonstrating higher interstitial volume, extracellular volume, and extraction flow in osteoporotic fractures compared to malignant fractures, with a specificity of 92%; however there were no significant differences in perfusion parameters in regions of increased MTT.35
Myeloproliferative Disorders and Malignancy
Perfusion imaging techniques can also be used to help differentiate normal marrow from marrow involvement by diffuse bone marrow disease, which can sometimes be difficult on anatomic imaging in young patients or adults with red marrow reconversion or in cases of early dissemination. Analysis of time-intensity curves in patients with aplastic anemia showed a lower peak value with slower washout compared to normal marrow.36 Myeloproliferative disorders including essential thrombocytopenia and polycythemia vera may demonstrate no significant differences in perfusion parameters compared to normal patients, although patients with myelofibrosis do demonstrate increased marrow perfusion.36,37
In hematologic malignancies, multiple myeloma, lymphoma, and metastatic disease, TIC analysis demonstrated earlier and steeper enhancement with faster wash-out of abnormal compared to normal marrow, even in areas where differences were not apparent on conventional MR images, probably related to neoangiogenesis.38,39 and 40 For patients with treated hematologic malignancies, the peak enhancement ratio has been shown to be significantly lower in patients in remission compared to those with recurrence.39 In patients with acute myelogenous leukemia (AML) in remission, perfusion parameters may help predict relapse-free and overall survival.41 Likewise, decreased perfusion parameters can indicate a treatment response for multiple myeloma and bone marrow metastases.42,43,44,45,46 and 47
▪ Osteonecrosis
Interest in early detection of acute avascular necrosis (AVN) was one of the first musculoskeletal applications of MR perfusion imaging, with lack of enhancement of avascular marrow demonstrated in a dog model before morphologic changes could be identified on routine MR sequences.48 Steroids have been implicated as one of the causative agents of osteonecrosis, and decreased femoral perfusion parameters after intravenous administration of a single dose of methylprednisolone in a rabbit model were demonstrated in 56% at 5 days and 83% at 14 days.49
In a study of patients with avascular necrosis of the femoral head, there was an increase in peak enhancement of the femoral
head with progressive osteonecrosis on DCE-MRI, although there was a delayed time-to-peak enhancement in patients with increased intramedullary pressure but normal MRI (grade 0) compared to controls.50 These findings are likely due to increased blood volume and reactive bone marrow edema with resultant stasis. Contrast-enhanced MRI may also help predict femoral head viability after femoral neck fracture, aiding the clinician in deciding between joint-preserving therapy versus arthroplasty.51
head with progressive osteonecrosis on DCE-MRI, although there was a delayed time-to-peak enhancement in patients with increased intramedullary pressure but normal MRI (grade 0) compared to controls.50 These findings are likely due to increased blood volume and reactive bone marrow edema with resultant stasis. Contrast-enhanced MRI may also help predict femoral head viability after femoral neck fracture, aiding the clinician in deciding between joint-preserving therapy versus arthroplasty.51
In patients with developmental dysplasia of the hip, global decreased enhancement after closed reduction is associated with a higher risk of AVN.52 In patients with systemic lupus erythematosus without AVN, femoral head perfusion was higher than healthy controls.53
Perfusion imaging can also be useful for assessing posttraumatic avascular necrosis. In a study of patients with scaphoid delayed union or nonunion, DCE-MRI of the proximal fragments with poor vascularity demonstrated lower peak enhancement and slopes than those with good vascularity, with strong correlation with surgical findings.54
▪ Paget Disease
Paget disease of bone is a disorder of bone turnover with cortical and trabecular thickening and elevated alkaline phosphatase.55 Pagetoid bone is hypervascular, especially along the advancing peripheral zone, and DCE-MRI can be used to evaluate regional microcirculation.56 MR perfusion imaging has also shown significant reduction in regional bone perfusion in Paget disease following bisphosphonate therapy.57
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree