Chapter 28 Myeloma and Leukemia
Myeloma
Epidemiology and Risk Factors
Multiple myeloma is a malignant disorder that results from a clonal proliferation of marrow plasma cells that usually results in the production of a monoclonal immunoglobulin in the serum or urine. Approximately 20,180 patients are diagnosed, and 10,650 die, annually in the U.S. with this disorder, making it the second most common hematologic malignancy in the United States.1 The disease most commonly occurs during the seventh and eighth decades of life, is more frequent in men (1.6:1), and has an incidence in African Americans over twice as high as that noted in whites.1
There is no undisputable risk factor for myeloma, but ionizing radiation or work in the paper, pulp, and leather tanning industries and other chemical exposures have been implicated.2 Numerous reports in people with chronic exposure to low-dose ionizing radiation exist, including radiology technicians/physicians prior to routine shielding and radium watch dial painters; perhaps the best evidence was among atomic bomb survivors at Hiroshima in an initial report, but the follow-up of this report failed to confirm those initial findings.3 Similarly, benzene has been implicated, but a thorough retrospective analysis of available reports eliminated this factor as well.4 Hereditary factors have not previously played a prominent role as risk factors for multiple myeloma, but recent reports suggest that at least among certain populations the incidence of monoclonal gammopathy of unknown significance (MGUS)/myeloma may have a familial/genetic association.5
Pathology
Myeloma begins with the clonal expansion of a malignant plasma cell that is usually positive for CD38, CD138, and monoclonal cytoplasmic immunoglobulin with a clonal light chain of either kappa or lambda type. These cells typically have an eccentric nucleus within a large cytoplasm and often have an area of central clearing that corresponds to intracytoplasmic immunoglobulin. Cells of plasmacytic origin are usually negative for other markers of the B-cell lineage such as CD19 and CD20. Recent studies suggest different categories of myeloma, which may separate disease into different levels of outcome.6 Although a heterogeneous array of cytogenetic abnormalities have been described in patients, deletions of chromosomes 13 or 17 p, certain translocations involving chromosome 14, on which the immunoglobulin H (IgH) locus resides [t(4;14), t(14;16)], and chromosome 1 abnormalities have predicted shortened survival; [t(11;14)] has usually been associated with either an average or an improved prognosis and rarely noted to have a subgroup with poor prognosis.6
The abnormal clonal proliferation usually results in the production of monoclonal immunoglobulin in the serum and/or urine; IgG is secreted in approximately 60%, IgA in 20%, IgD in 2%, and IgE in less than 1%; biclonal secretion is rare. Secretion of light chain as the sole monoclonal protein is noted in 18%.7 Previously, 3% of patients were noted to have nonsecretory disease; this number has declined with the advent of serum free light chain (kappa, lambda free kappa:lambda) detection.
Clinical Presentation
Bone disease occurs in nearly 70% of newly diagnosed patients with multiple myeloma and results, in part, from RANKL overexpression and OPG inhibition resulting in unbridled activation of osteoclasts. Other cytokines such as interleukin-1beta (IL-1β), IL-6, and tumor necrosis factor-alpha (TNF-α) also have a role in lytic bone disease.8,9 Progressive bone destruction results in hypercalcemia in approximately 20% of patients with newly diagnosed myeloma.
Marrow infiltration with plasma cells results in a normocytic, normochromic anemia in the majority of patients with previously untreated myeloma. Recently, up-regulation of hepcidin mRNA in myeloma patients has been noted and may play a causative role in this complication.10 In patients with high levels of circulating monoclonal serum protein, stacking of red blood cells on the peripheral blood smear, known as rouleaux formation, may occur.
Nearly one half of patients will develop renal failure at some time during the course of their disease. Cast nephropathy of the distal tubule is the most common cause of this complication, but hypercalcemia, dehydration, hyperuricemia, and concomitant conditions such as amyloidosis and light or heavy chain deposition diseases may also be contributing factors.11
Frequent bacterial infections may also be noted in patients with myeloma due to suppression of uninvolved immunoglobulins, decreased antibody response, and impaired opsonization among other abnormalities reflective of the impaired immune response in patients with multiple myeloma.12,13
Although the diagnosis of myeloma by fixed criteria may be difficult, the International Myeloma Working Group (IMWG) proposed a simple system based on the presence of 10% or greater clonal marrow plasma cells and/or the presence of a serum monoclonal protein 3.0 g/dL or greater.14 The same group developed the “CRAB” (HyperCalcemia, Renal Failure, Anemia, Bone lesions) criteria to determine whether there has been end-organ damage that would classify a patient as having symptomatic disease that would require initiation of treatment.14 These criteria include elevated serum Calcium (≥11.5 mg/dL), Renal insufficiency (creatinine > 2 mg/dL), Anemia (>2 g/dL below the lower limit of normal or < 10 g/dL), Bone lesions (lytic disease or osteoporosis with compression fractures), or other complications (hyperviscosity, amyloidosis, and light chain deposition diseases). Patients without any of these complications are considered asymptomatic and may be observed, without treatment, with frequent follow-up until progression to symptomatic disease.
It is important to distinguish multiple myeloma from related plasma cell dyscrasias such as MGUS and solitary bone plasmacytoma (SBP) or extramedullary plasmacytoma (EMP). The diagnosis of MGUS relies on the presence of fewer than 10% marrow clonal plasma cells and a monoclonal serum protein of less than 3 g/dL in a patient who has no evidence of end-organ/tissue damage, including anemia, hypercalcemia, renal failure, or lytic bone lesions attributable to myeloma and no evidence of a B-cell proliferative disorder.14 SBP or EBP is diagnosed when a single lesion of biopsy-proven clonal plasma cells is detected without evidence of other disease including negative magnetic resonance imaging (MRI) of the spine and bone marrow examination; it is particularly important to identify these patients because 35% to 65% may be curable when given radiation therapy with curative intent.15
Key Points Monoclonal gammopathy of unknown significance
<10% clonal marrow plasma cells
AND serum monoclonal protein less than 3.0 g/dL
• Single lesion of biopsy-proven clonal plasma cells without other evidence of myeloma including negative MRI of the spine and negative bone marrow aspiration.
• Meets criteria for myeloma but without any of the complications listed below for classification as symptomatic myeloma.
Staging
Numerous factors have been suggested to determine the prognosis of patients with myeloma. In the 1970s, Durie and Salmon16 proposed a staging system based on the degree of anemia, level of paraprotein, presence or absence of hypercalcemia, lytic lesions, and renal insufficiency that subsequently became the standard staging system for myeloma for several decades (Table 28-1). Although this system was predictive of the degree of myeloma tumor mass, several components were imprecise, such as extent of bone lesions and the inclusion of factors such as anemia, hypercalcemia, and renal function, which may be affected by factors other than myeloma infiltration. Subsequently, multiple new prognostic factors emerged including plasma cell hypodiploidy, C-reactive protein, lactate dehydrogenase, labeling index, cytogenetic abnormalities, and beta-2 microglobulin (β2M).
In 2005, Greipp and coworkers17 proposed the International Staging System (ISS) that separated patients into three stages based on the level of serum albumin and β2M. The data from 10,750 patients were analyzed by univariate and multivariate analysis; the system was designed using half the data set and validated in the other half of the population (Table 28-2). This system effectively identified a group of patients with a relatively short median survival of 29 months (β2M ≥ 5.5 mg/L, stage III), another with longer median survival of 62 months (β2M < 3.5 mg/L and albumin > 3.5 g/dL, stage III), as well as a group with intermediate survival (stage II). Although some patients in the data set may have received thalidomide, the impact on survival of bortezomib, lenalidomide, and multiple combinations utilizing both of these drugs will likely lead to improved median survivals for one or more stages defined by the ISS; the Greek Myeloma Study Group also confirmed the validity of the ISS in the era of novel agents.18 The ISS has subsequently become a standard staging system for patients with myeloma.
Table 28-2 International Staging System for Multiple Myeloma
| STAGE | CRITERIA |
|---|---|
| 1 | β2M < 3.5 mg/L Albumin ≥ 3.5 g/dL |
| 2 | β2M 3.5-5.5 mg/L or β2M < 3.5 mg/L and Albumin < 3.5 g/dL |
| 3 | β2M > 5.5 mg/L |
β2M, beta-2 microglobulin.
From Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23:3412-3420.
Although the Durie-Salmon system and ISS effectively separate patients into different survival categories, the impact of chromosomal abnormalities on prognosis is significant, and some have even suggested a molecular classification of myeloma.6 Certain translocations involving chromosome 14 [t(4;14), t(14;16)] and deletion of chromosome 13 have previously predicted a poor prognosis, but treatment with bortezomib appears to overcome the prognostic impact of these abnormalities; the same data with lenalidomide are premature and need to be validated over time.6,19,20 Deletion of chromosome 17p remains prognostic even in the era of novel agents; other considerations include the poor prognosis of chromosome 1 abnormalities and the possibility of an improved prognosis with t(11;14).6 Although no staging system based on chromosomal abnormalities has been specifically agreed upon, the aforementioned considerations are essential for the determination of prognosis and appropriate treatment decisions.
Imaging
Goals
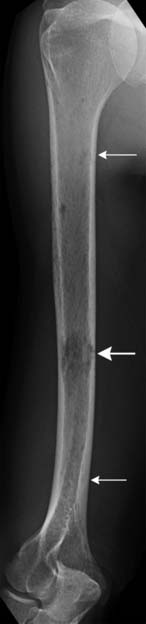
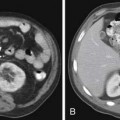
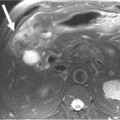
When a patient is suspected of having an isolated plasmacytoma, imaging initially serves two roles. The first is similar to its role in any focal solid tumor—to define the anatomy of the tumor and its relationship to other structures in order to assist with local treatment planning, which will usually be radiation therapy with curative intent. The second is to determine whether the seemingly isolated plasmacytoma is the only site of disease or whether it is merely the tip of the iceberg in a patient who should properly be classified as having multiple myeloma. To that end, one may weigh and choose between the various forms of systemic imaging, to be discussed later. If no other tumor is found elsewhere, and the patient remains classified as having solitary plasmacytoma, then follow-up imaging also serves two purposes. The first is again similar to its role in other solid tumors—to evaluate the site of the primary disease for evidence of healing or, alternatively, recurrence or progression (Figure 28-1). The second is surveillance for development of multiple myeloma, again by use of systemic imaging along with other criteria.
For patients with multiple myeloma, imaging helps at initial evaluation to distinguish those with asymptomatic or smoldering myeloma, who have no visible bone disease, from those with symptomatic myeloma. Once the patient has been categorized into the symptomatic or asymptomatic group, imaging is used to assess stability versus progression of disease.
Key Points Radiology report
• Identify and characterize focal bone lesions: Number, location, and size when practical. (For multiple lesions, it is often impractical to discuss each separately.)
• Clearly indicate when a bone lesion is thought due to disease other than myeloma, such as a geode due to degenerative joint disease.
• Discuss specific lesions with significantly elevated risk of pathologic fracture.
• Assess subjective bone mineralization—normal, decreased absolutely, decreased allowing for age and gender.
• Look for and discuss compression fractures in the spine.
• Follow-up examinations: Indicate whether the disease is stable or progressive or shows signs of healing to include resolution of fluoro-2-deoxy-D-glucose (FDG) avidity at positron-emission tomography/computed tomography (PET/CT) or decrease in contrast enhancement at MRI.
Techniques
Conventional Radiography
Skeletal surveys have for decades been a mainstay of imaging in multiple myeloma and related diseases and are included in the original Durie and Salmon staging system.16 They consist of a series of images intended to include all the bones that have a reasonable likelihood of developing visible signs of multiple myeloma. There is no generally agreed-upon grouping of images to be included in a skeletal survey, and so there will be variation from one institution to another as to exactly what is included. Based on observation not only of our own practice pattern but also of the assortment of images obtained at outside institutions and then reviewed within our institution, the minimum number and type of views might reasonably be lateral skull, lateral cervical spine, frontal and lateral thoracic and lumbar spine, frontal chest or ribs, frontal pelvis, and frontal views of each humerus and femur. That would be 12 views. The assortment of images suggested by the IMWG21 is listed in a key point text box later. Whole body radiography has been suggested as an alternative to the individual views obtained with skeletal surveys.22
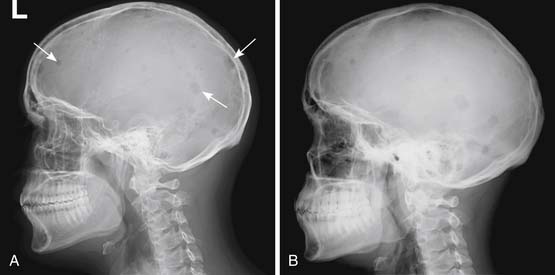
Skeletal surveys have the advantage of being relatively cheap and easy to perform using widely available equipment. They are adequate for finding lytic bone lesions that have reached a sufficient size. Generally that means loss of 30% to 50% of the bone mineralization.23,24 For an individual lesion, the size needed for it to be visible will depend greatly on which bone is involved and where the lesion is in the bone. For example, if a femoral lesion is located along the lateral or medial margin of the bone, it will erode the endosteal surface of the cortex and create a scalloped border that will be noticeable earlier than a similarly sized lesion located either anteriorly or posteriorly, assuming that the bone is being imaged in the frontal projection. Lesions in the pelvis and sacrum often have to attain a size of several centimeters before they are noticed because overlying bowel gas may mask them. In the skull, the presence of normal lucencies due to venous lakes can make it difficult to decide whether a particular lucent area is normal or abnormal.
Skeletal surveys are obtained as part of the initial diagnostic workup of patients with MGUS or suspected SBP because the presence of unexpected lytic bone lesions would necessitate recategorization, probably to multiple myeloma. Skeletal surveys are also obtained in patients with myeloma to help determine the stage of disease when diagnosed. If the skeletal survey shows lytic lesions, more advanced imaging may be unnecessary. The skeletal survey is also useful for searching for progression after treatment.25
Skeletal surveys and other conventional radiographs can reveal disease progression but are of limited utility in assessing response to therapy because lytic bone lesions heal very slowly, if at all24 (Figures 28-2 and 28-3).
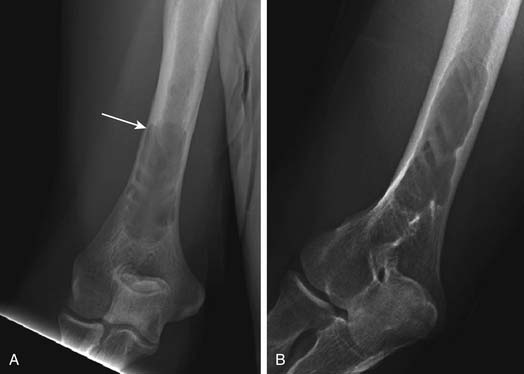
Another use of conventional radiography is in assessing the risk of pathologic fracture once a lytic lesion has been identified by any means. For this one usually wants both frontal and lateral views of the area in question (Figure 28-4).
Computed Tomography
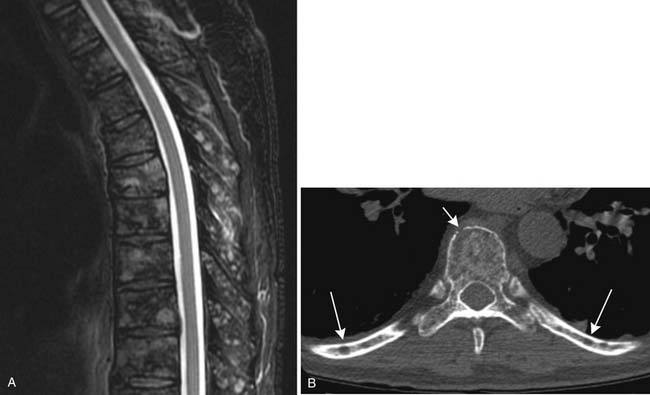
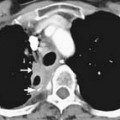
CT provides cross-sectional radiographic images of the body and can serve many purposes. Myeloma’s most common anatomic manifestation is in the bones. CT can demonstrate smaller lytic lesions than would be apparent by conventional radiography and can, like conventional radiography, help estimate the risk of pathologic fracture21–23 (Figure 28-5). With attention to the difference in attenuation between tumor (water density) and marrow (fat density), CT can also demonstrate marrow involvement. Despite these capabilities, CT is not commonly used for evaluation of osseous myeloma, although low-dose whole body CT has been suggested as an alternative to the skeletal survey.26 CT is also quite useful for detection and characterization of the uncommon soft tissue manifestations of myeloma or complications of its treatment.
Magnetic Resonance Imaging
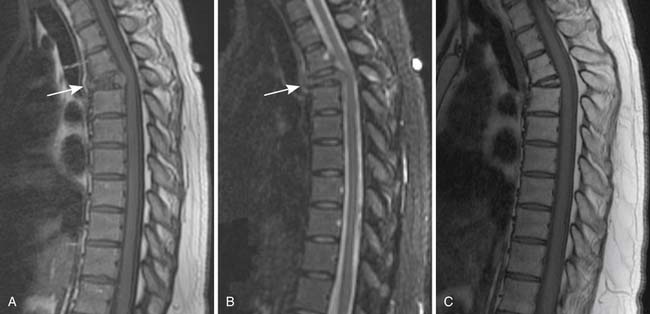
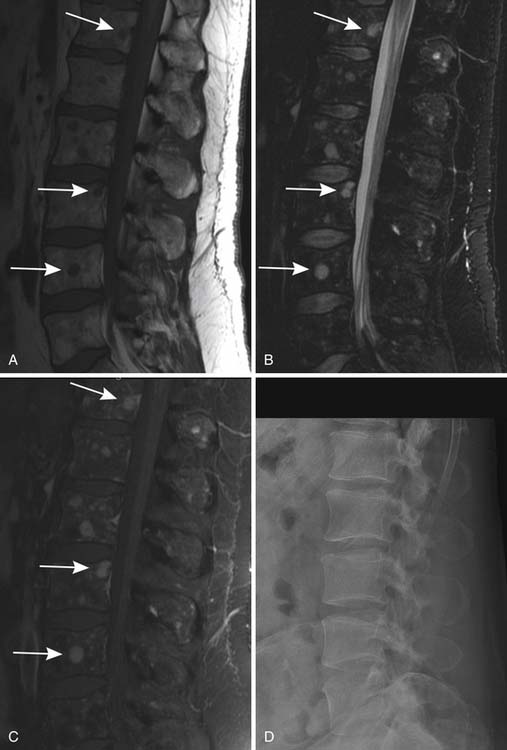
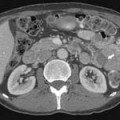
MRI is also a cross-sectional imaging technique that differs from CT in having inherently lower spatial resolution but greater contrast resolution, an advantage in imaging the bone marrow. Because of this, its most typical use in myeloma is in staging assessment of bone marrow involvement. Myeloma can involve any bone, but for assessing a large amount of bone marrow in one MRI examination, spine MRI has been traditionally used for myeloma assessment. With the advancement of new techniques using coils adapted to image large areas, imaging from the skull base to the proximal thighs can be done, including assessment of the entire body in one scan. This whole body MRI examination gives a very broad view of the marrow at the cost of spatial resolution. Whole body MRI has recently been tested as a staging method for plasma cell neoplasms. Ghanem and colleagues27 compared whole body MRI and skeletal surveys in patients with either myeloma or MGUS. Among 54 patients, whole body MRI found bone marrow involvement in 10 who had negative skeletal surveys. Walker and associates28 also found MRI to be more sensitive to focal bone lesions than skeletal survey. Evaluating 100 patients with MGUS or myeloma, Bäuerle and coworkers29 compared spinal MRI with whole body MRI and found 9 patients with isolated extra-axial disease that was identified only with whole body MRI.
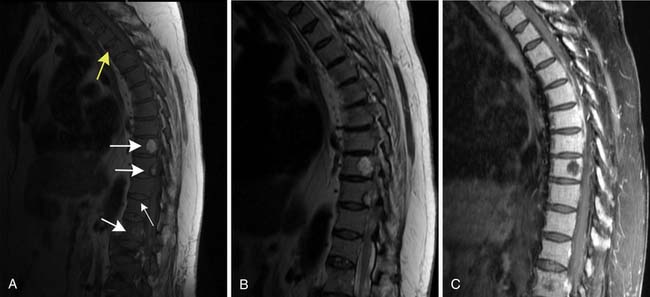
Patterns of myeloma involvement seen on MRI include normal marrow (which does not exclude low-level infiltration of myelomatous cells), focal involvement (Figure 28-6), diffuse homogeneous involvement (Figure 28-7), and heterogeneous involvement, with combinations of fatty marrow and tiny areas of myeloma creating a salt-and-pepper appearance.30–32 Shorter time to progression and worse response to therapy have been found with diffuse and focal disease as opposed to normal marrow or heterogeneous involvement.32–34 With successful treatment, the marrow pattern should become more normal.24
Positron-Emission Tomography
The purpose of PET/CT varies depending on the stage of disease. In newly diagnosed patients, it helps find sites of disease, both osseous and in the soft tissues, that may be occult by conventional radiography. In this way, it can help distinguish a solitary plasmacytoma from multiple myeloma.35 Kim and colleagues36 found PET to be positive in 13 of 14 known plasmacytomas, and in 1 of 21 patients with suspected SBP, PET imaging resulted in upstaging of the disease to multiple myeloma by disclosing previously unsuspected additional disease. Depending on the equipment available, PET/CT will usually cover at least the head, trunk, and proximal humeri and femurs, and it may be able to include literally the entire body, so like large–field of view MRI, it is a way to include most or all of the body in a more sensitive screening than is available with a bone survey. In patients who have been treated, PET/CT is used to identify sites of disease progression and to distinguish the osseous lesions that are still apparent anatomically but are no longer metabolically active from those that are resistant to treatment and remain active37,38 (Figure 28-8). Complete suppression of abnormal PET activity after treatment may be linked to longer progression-free survival (PFS) than for patients without complete suppression.39 As compared with MRI, PET/CT tends to be less sensitive to diffuse marrow infiltration and perhaps more sensitive to the presence of focal lesions.40
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree