▪ Introduction
Coronary artery disease (CAD) remains a leading cause of morbidity and mortality worldwide.
1 The natural history of CAD is characterized by the silent accumulation of atherosclerotic plaque in the wall of coronary arteries, progression into obstructive disease as excess plaque accumulates beyond maladaptive positive remodeling, the development of stress-induced ischemia as a result of luminal encroachment, and ultimate plaque rupture resulting in unstable angina, myocardial infarction, and sudden cardiac death.
2Over the past decade, multiple studies have demonstrated that coronary CT angiography (CCTA) is a robust noninvasive method for detection of atherosclerotic plaque and obstructive CAD (defined as the presence of >50% stenosis on invasive angiography) with a high sensitivity and negative predictive value in patients with suspected CAD.
3,
4,
5 and
6 Accordingly, since 2010, CCTA has been recommended by the American College of Cardiology as an appropriate upfront investigation for symptomatic patients with low and intermediate risk of CAD.
7,
8 The use of CCTA in this population is currently a class IIa recommendation according to European Society of Cardiology and American College of Cardiology guidelines.
9,
10While CCTA accurately detects anatomical disease, in its current form, it is limited in predicting lesion-specific ischemia.
11,
12 When compared with invasive fractional flow reserve (FFR), which is the current gold standard for determination of vessel- and lesionspecific ischemia, CCTA has been found to have a high sensitivity and negative predictive value for ischemia, yet the specificity and positive predictive value are significantly limited (
Table 21.1). This is a notable limitation as myocardial ischemia represents an important factor that determines clinical outcomes
21 and benefits from revascularization.
22,
23,
24 and
25 For this reason, patients with identified significant stenoses on CCTA often require further functional assessment, which entails additional testing, radiation exposure, cost, and inconvenience.
Current assessment for myocardial ischemia is typically performed with noninvasive stress imaging including stress echocardiography, single photon emission computed tomography (SPECT), or magnetic resonance imaging (MRI) myocardial perfusion imaging (MPI). It should be noted that with the exception of MRI MPI, which is commonly available only in quaternary referral centers,
19,
26 stress echocardiography and SPECT MPI have been demonstrated to have poor discrimination of vessel-specific ischemia.
27,
28 and
29 Using invasive FFR as reference standard for vessel-specific ischemia, SPECT MPI identifies ischemia territory correctly less than 50% of the time, with underestimation and overestimation in 36% and 22% of cases, respectively.
27 Such data have evoked concerns for the ability of stress testing to effectively isolate coronary lesions that benefit from revascularization (
Table 21.2).
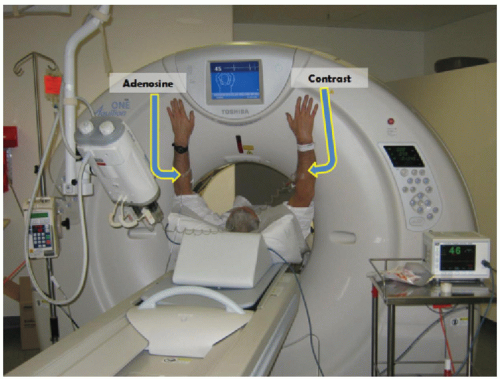
Over the past 5 to 10 years, an important focus of CT research is the development of novel techniques to evaluate each step in the pathophysiology of CAD including the assessment of the hemodynamic significance of coronary artery lesions and associated myocardial ischemia (
Fig. 21-1). These are aimed at enabling cardiac CT to act as a one-stop shop modality to assess the anatomical and physiologic sequelae of CAD. The novel CT techniques, which will be discussed in this book chapter, include CT stress myocardial perfusion imaging (CTP) to evaluate for the presence of myocardial ischemia, the use of noninvasive CT fractional flow reserve (FFR CT), and the study of transluminal attenuation gradient (TAG) across coronary lesions to assess the hemodynamic significance of coronary lesions.