Risk factors associated with the development of osteonecrosis
1. Traumatic conditions
2. Nontraumatic conditions
(a) Corticosteroid use (dose >0.5 mg/kg)
(b) Alcohol abuse
(c) HIV
(d) Radiation therapy
(e) Systemic lupus erythematosus
(f) Sickle cell disease (SS or SC or Sβ+)
(g) Dyslipidemia (hypertriglyceridemia)
(h) Caisson disease
(i) Gaucher’s disease
(j) Fat embolism
(k) Organ transplantation (glucocorticoid mediated mostly)
(l) Endogenous hypercorticolism (rare)
(m) Renal failure
(n) Pancreatitis
(o) Cancer chemotherapy
(p) Idiopathic
(q) Hypercoagulable conditions (proteins C and S and antithrombin III deficiency)
The disruption of the vascular supply in osteonecrosis can result from extra- (e.g., elevated marrow pressure, increased marrow fat) or intraluminal vascular obstruction (e.g., microscopic emboli, sickle cells, nitrogen bubbles) or focal clotting abnormalities (e.g., proteins C and S or antithrombin III deficiency) leading to reduction in the blood flow. Various genetic factors (e.g., polymorphisms of endothelial nitric oxide synthetase) and cytotoxic factors have also been postulated to play a role in its development. When this condition affects the subchondral region, it often leads to subsidence of the overlying articular cartilage causing joint incongruity and subsequent development of end-stage arthritis. Although the vascular disruption is obvious in traumatic cases, the underlying etiopathogenesis in nontraumatic osteonecrosis is often not clear. It is commonly agreed upon that once extensive collapse occurs, total joint arthroplasty remains the only reasonable treatment option. The following sections provide an overview of the natural history of both symptomatic and asymptomatic disease.
20.2 Natural History of Symptomatic Osteonecrosis
The natural history of symptomatic osteonecrosis and its progression is probably better understood than the multitude of early triggering factors that are responsible for its development. It is often surprising that in some patients osteonecrosis often develops within 1–6 months of exposure to a known risk factor, while in others it never develops despite being exposed to the same risk factor. Analysis of the natural history of symptomatic disease provides a basis for comparison of the efficacy of treatment interventions in nonrandomized studies, allows patients to be informed about prognosis and expected outcomes, and enables assessment of whether nonoperative or surgical treatment will provide better outcomes in a given clinical scenario.
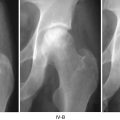
Previous studies have reported that symptomatic osteonecrotic lesions occurring in the weight-bearing regions of the femoral head have a high rate of collapse. Moreover, it was also found that development of subchondral fracture was an early predictor of future collapse (Ficat-Arlet stage III and Steinberg stage IV). Articular surface collapse is considered an irreversible turning point in the natural history of osteonecrosis. This is strongly associated with pain, future development of osteoarthritis, and a poor eventual outcome. It has been found in various studies that once radiographic diagnosis of osteonecrosis is made, collapse usually occurs within 2 years in 32–79 % of patients. Shimuzu et al., in a MRI study, evaluated the risk of progression to collapse for early-stage lesions in 66 hips [1]. At a mean follow-up of 49 months, the authors found that 74 % of the hips that had osteonecrotic involvement of at least 25 % of the head diameter stage I lesions had progressed to stage II disease, while 21 % had progressed beyond stage II. Forty-eight percent of the hips that had stage II disease on presentation had progressed to stage III at final follow-up.
It was initially believed that if no radiographic progression occurs after 3 years from diagnosis, further risk of deterioration is considered to be low. However, Hernigou et al. reported delayed progression of pain and collapse in lesions involving less than 10 % of the femoral head, at an approximate follow-up of 7 years. This suggests that there may be a possibility of inevitable progression of disease once radiographic diagnosis is made. In a separate study Hernigou et al. evaluated the natural history of 92 symptomatic hips in 64 sickle cell patients, at a mean follow-up of 17 years [2]. Of these 92 hips, there were 32 hips in Steinberg stage I, 43 in stage II, 2 in stage III, and 15 in stage IV disease. The authors found that 65 of the 75 hips (87 %) with radiographic evidence of disease on diagnosis sustained collapse within 5 years of diagnosis. The mean time to collapse from diagnosis was 30 months for stage II hips and 42 months for hips in stage I. The authors also found that the size of the lesion had a significant effect on the duration of survival, with mild involvement having a longer survivorship compared to moderate or severe involvement irrespective of the stage of the disease (p = 0.01).
Similarly, poor outcomes were reported by Gutierez et al. who in a multicenter study evaluated the natural history of 54 symptomatic osteonecrotic hips secondary to HIV infection. The authors reported that 37 % (20 out of 54 hips) of patients had clinical progression of the disease requiring surgical intervention at a median interval of 12.7 months following diagnosis. The authors also found that male sex (p = 0.03) and a relatively high CD4 (p = 0.03) were significantly associated with the risks of disease progression requiring surgery.
Improvement of radiographically obvious lesions is exceedingly rare and is currently controversial. Limited studies have evaluated the changes in dimension of osteonecrotic lesions. A few studies have reported that in 23–45 % of cases, a moderate decrease in size of the lesions can occur. Kopecky et al. in a study of 104 renal transplant recipients found MRI changes in 25 hips [3]. During follow-up evaluation these changes were found to reduce in 7 hips and improve in 6 hips. Other authors have reported no progression in the size of the lesions [1, 4]. However, none of the studies were designed to evaluate changes in lesion size over time.
In summary, multiple factors influence the natural history of symptomatic osteonecrosis. A slightly more favorable prognosis occurs in patients who have no radiographic abnormalities at presentation compared to patients who present with radiographic stage II lesions, although the general consensus is that once clinical and radiographic evidence of osteonecrosis occurs, gradual progression to collapse and development of arthritis occurs if no intervention is undertaken. Most authors believe that size has a profound effect on the prognosis of these lesions. Small lesions involving less than 10 % of the head have better outcomes in terms of longer survival and less chance of progression, compared to lesions that involve more than 25 % of the head size. In addition, the proportion of involvement of the weight-bearing area (stage A, <1/3rd; stage B, 1/3rd to 2/3rd; stage C, >2/3rd) of the head affects the rate of progression to collapse. It has been found that the rate of progression to Ficat-Arlet stage III varies from 0 % in stage A and increases to 70 % with stage C disease. Moreover, risk factors such as alcohol abuse, corticosteroid use, presence of radiographic evidence of effusion, and marrow edema underlying the necrotic region have been associated with increased risks of disease progression [5]. Current evidence suggests that size of the lesion usually remains stable over time, although a modest decrease in size can occur. However, complete resolution of osteonecrotic lesions is extremely rare.
20.3 Natural History of Asymptomatic Disease
Many patients are diagnosed when the disease presents itself with gradual onset of pain in the hip, buttock, or groin region which often increases on weight bearing, at least initially, and then progresses to constant rest pain. However, during screening of at-risk populations, often asymptomatic disease is found on the contralateral side. The progression of asymptomatic disease is often uncertain leading to some surgeons recommending a “watchful-waiting” policy for small- or medium-sized lesions, while others believe that many of these asymptomatic patients will ultimately progress and early treatment with joint-preserving procedures may forestall further progression and future need for a total joint arthroplasty. Still others believe that an individualized approach based on various risk factors (e.g., demographic, epidemiological, and radiographic) for disease progression may be needed, rather than a “blanket approach” for treatment of these lesions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree