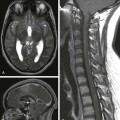
Chapter 16 Imaging is frequently used to evaluate neck tumors in pediatric patients. The choice of imaging modality depends on tumor location and treatment options. Ultrasound with Doppler distinguishes cystic lesions from solid lesions, detects venous or arterial vascularity, and differentiates nodal masses from nonnodal masses. Computed tomography (CT) characterizes bony changes and detects intralesional calcification. CT images are acquired as helical axial thin sections using a split-dose bolus of intravenous (IV) contrast (half of the IV bolus is administered, and images are acquired after a 3-minute pause during the administration of the second half of the bolus). Multiplanar reformatted images with bone and soft tissue reconstruction algorithms should be provided. The lowest radiation dose that produces diagnostic quality images should be used. Magnetic resonance imaging (MRI) demonstrates the soft tissue characteristics of the tumor. The MRI protocol should include multiplanar T1, fat-suppressed T2 or short-T1 inversion recovery (STIR) images, a flow-sensitive gradient echo sequence, diffusion-weighted images, and IV gadolinium-enhanced, fat-suppressed, T1-weighted sequences. Nuclear medicine studies including fluorine-18-fluorodeoxyglucose positron emission tomography (18F-FDG PET) and PET CT are used for staging and follow-up of various tumor types, particularly lymphoma. The following features are important with regard to the differential diagnosis: patient age, clinical history, physical examination, tumor location, and characteristic imaging features (e.g., mineralization, vascularity, and intensity of enhancement). The most common benign tumor is hemangioma. Lymphoma (approximately 50% of cases) and rhabdomyosarcoma (RMS) (approximately 20% of cases) account for most malignant pediatric head and neck tumors.1,2 Thyroid, nasopharyngeal, and salivary gland carcinomas are the most frequently seen pediatric head and neck carcinomas. Overview: Hemangioma is the most common vascular tumor that occurs in infants, more commonly in girls. Infantile hemangiomas proliferate during the first year of life and then involute over the next several years. Proliferating hemangiomas display prominent vascularity. With involution, progressive decrease in size and vascularity and an increase in fibrofatty matrix occur. True congenital hemangiomas are uncommon and are characterized as either rapidly involuting congenital hemangioma or noninvoluting congenital hemangioma. Approximately 20% of children have multiple hemangiomas. Hemangioma that involves skin produces a raised, knobbly, scarlet-colored mass or sometimes a macular lesion that can be confused with a port wine stain in the neonate. Complications of hemangiomas include deformity, mass effect, interference with vital functions, ulceration, and bleeding. The acronym PHACES describes the association of posterior fossa malformations, hemangiomas, arterial anomalies, coarctation of the aorta and cardiac defects, eye abnormalities, sternal malformations, and supraumbilical raphe.3,4 The hemangiomas are typically large plaque-like or regional cutaneous hemangiomas of the face, sometimes in the midline or appearing beard-like. The most common posterior fossa anomaly is mild unilateral cerebellar hypoplasia with prominence of the ipsilateral retrocerebellar cerebrospinal fluid space. Anomalies of the internal carotid and vertebral arteries include absence or hypoplasia, tortuosity, and ectasia or aneurysmal change, and persistence of the trigeminal artery. Progressive cerebrovascular occlusive changes with moyamoya phenomenon have also been described in patients with craniofacial hemangioma.5 Imaging: Proliferating infantile hemangioma during the first year of life has characteristic imaging features. The tumors are solid, circumscribed, lobulated, and highly vascular by ultrasound, CT, and MRI (Fig. 16-1). Hemangiomas are isodense with muscle and enhance rapidly and intensely following the administration of contrast agent on CT. Prominent vascular signal voids on spin-echo sequences on MRI or flow-related enhancement on magnetic resonance angiography is typical. Proliferating hemangiomas are moderately hyperintense relative to muscle on T2-weighted images and enhance intensely. Involuting hemangiomas have reduced vascularity and enhancement. True intraosseous hemangioma (as opposed to osseous venous malformations, which are often erroneously referred to as “hemangioma”) and small or involuting hemangiomas can be more difficult to diagnose with certainty. The differential diagnosis for hemangioma includes pyogenic granuloma, which is usually superficial and infrequently imaged. Poorly defined or fuzzy margins are atypical for hemangioma and should prompt an alternative diagnosis such as kaposiform hemangioendothelioma. Other rare congenital tumors such as rhabdomyoma and RMS lack prominent vascularity. Infantile fibrosarcoma is sometimes somewhat vascular and hemorrhagic but usually causes more aggressive bony erosion and does not enhance as intensely as proliferating hemangioma. Hemangioma within or adjacent to bone occasionally produces bony remodeling and corticated bony erosion simulating Langerhans cell histiocytosis, but aggressive lytic bony destruction should prompt consideration of a malignant neoplasm. Figure 16-1 Proliferating infantile hemangioma. Management: Hemangiomas are usually treated expectantly, anticipating involution. However, if interfering with vital functions, accelerated involution is promoted by medical therapy with steroids, alpha-interferon, or, more recently, propranolol.6 Laser therapy and embolization are therapeutic options for selected cases. Overview: Teratoma is the most common congenital tumor of the neck and is the second most common location of teratoma in early infancy. Histologic immaturity in congenital teratoma does not necessarily reflect an adverse outcome, as is seen in adolescents and adults. Imaging: Prenatal diagnosis of teratoma is made with fetal ultrasound or MRI. The tumor is sharply circumscribed, and heterogeneous, with solid and cystic areas. Compression of the airway by tumor may affect the timing and mode of delivery and should prompt delivery of the baby by the fetal exit procedure with elective cesarian section and securing of the airway while the neonate is receiving placental support. Midline teratoma that involves the oral cavity prevents normal development and apposition of the palatal shelves, resulting in cleft palate. Infrahyoid teratoma frequently involves the thyroid gland and is considered by some to be of thyroid origin.7 On ultrasound, CT, and MRI, teratomas appear heterogeneous, with solid and cystic components (Fig. 16-2). Flecks of calcification and fat are characteristic. Other than fat, the soft tissue components typically enhance. The differential diagnosis for predominantly cystic teratoma is lymphatic malformation (LM). The presence of calcification and the apparent origin of the tumor from the thyroid gland with absence of the ipsilateral thyroid lobe or splaying of thyroid tissue around the tumor periphery are key features that distinguish teratoma from LM. Other neck tumors that contain fat include lipoma and lipoblastoma. The most common calcified tumor in the neck is pilomatrixoma, which is small, calcified, and cutaneous or subcutaneous. Less common congenital solid tumors that can be confused with teratoma include infantile myofibromatosis and kaposiform hemangioendothelioma with lymphomatoid components. Figure 16-2 Congenital teratoma of the neck in a newborn boy. Imaging: Solitary neurofibroma, plexiform neurofibroma, and malignant peripheral nerve sheath tumor (MPNST) usually occur in children with neurofibromatosis (NF) type 1. Schwannoma occurs sporadically or in patients with NF2. Solitary neurofibroma and schwannoma are well marginated and of variable signal on T1- and T2-weighted images (Fig. 16-3, A). Fibrous tissue in neurofibroma and Antoni A tissue in schwannoma produce T2 shortening that can simulate high-grade cellular neoplasms. These nerve sheath tumors enhance, and cystic foci can be seen in schwannoma. Plexiform neurofibromas appear multinodular or as worm-like masses extending along peripheral nerves or nerve roots, sometimes within multiple fascial compartments. Plexiform neurofibroma appears hypodense on CT and hyperintense relative to muscle on T2-weighted MRI and has a characteristic “target sign” on CT and central punctuate hypointensity on T2-weighted MRI (see Fig. 16-3, B). This feature helps distinguish this tumor from microcystic LM, which also tends to be transspatial across anatomic neck compartments. MPNST is heralded by pain, rapid increase in size of a peripheral nerve sheath tumor, change in enhancement characteristics, or the development of metastases (see Fig. 16-3, C). FDG PET imaging is useful for evaluation of suspected MPNST.
Neoplasia
Hemangioma
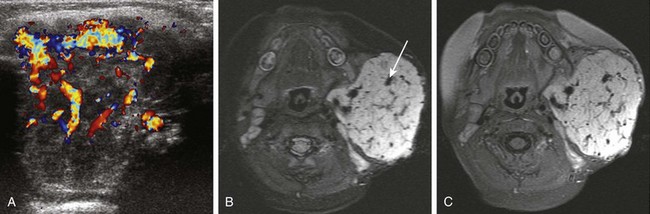
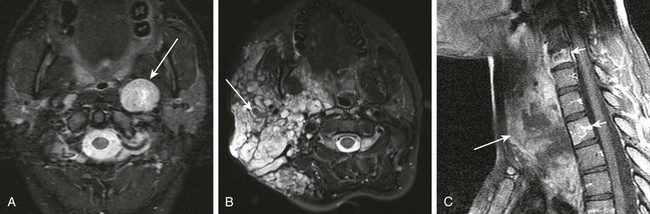
A, A 4-month-old girl with a parotid mass. Color Doppler ultrasound reveals a large, moderately echogenic hemangioma with prominent vascularity. B, Three-month-old baby boy with a parotid mass. Axial fast spin-echo inversion recovery magnetic resonance (MR) image shows a large, sharply circumscribed hemangioma with prominent vascular flow voids (large arrow). The mass is of slightly higher signal intensity than the spinal cord. C, Axial fat-suppressed T1-weighted MR image with intravenous contrast in the same patient demonstrates intense enhancement of the hemangioma.
Teratoma
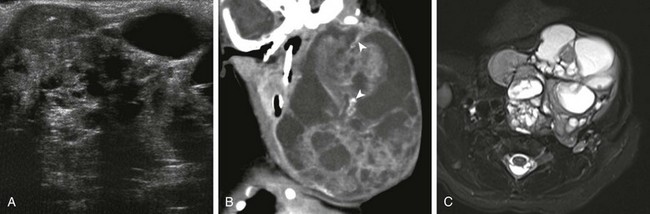
A, Ultrasound of the neck shows a heterogeneous cystic and solid teratoma. B, Reformatted coronal computed tomography image with intravenous contrast shows that the cystic and solid teratoma contains flecks of calcification (arrowheads). C, Axial fast spin-echo inversion recovery magnetic resonance (MR) image shows the cystic and solid teratoma.
Nerve Sheath Tumors