Functional and physiologic
Follicles
Hemorrhagic
Corpus luteum
Inflammatory
Pelvic inflammatory disease
Endometrioma
Other benign
Paratubal cysts
Hydrosalpinx
Ectopic pregnancy
Ovarian torsion
Benign neoplasms
Germ cell
Mature cystic teratoma
Sex cord–stromal
Fibroma
Epithelial
Serous or mucinous cystadenoma
Malignant neoplasms
Germ cell tumor
Dysgerminoma
Immature teratoma
Sex cord–stromal tumor
Granulosa cell tumor
Epithelial ovarian carcinoma
Borderline or low malignant potential
Invasive epithelial
Fallopian tube carcinoma
The latter category can be split into the following sub-categories:
(a)
Surface epithelial–stromal tumors
(b)
Sex cord–stromal tumors
(c)
Germ cell tumors
(d)
Secondary (metastatic) tumors
These latter are also important as treatment options differ from those for primary ovarian malignancies.
Overall, it is widely accepted that if an adnexal mass is considered to have an appreciable risk of malignancy, surgery should be recommended. It is nevertheless crucial to consider the clinical context of each individual patient diagnosed with an adnexal mass when determining the most appropriate diagnostic and therapeutic strategy. Partly because of the low incidence of ovarian cancer in the general population, there are no accepted effective screening tests to early identify women with ovarian cancer [4] – unless there is a strong family history or a known gene mutation (e.g., BRCA).
There are three main clinical routes by which an adnexal mass may be detected: (1) symptomatic women diagnosed with an adnexal mass by physical exam or imaging, (2) adnexal mass detected during bimanual pelvic examination, and (3) asymptomatic adnexal mass detected incidentally on imaging performed for another indication [2].
In premenopausal women, the most likely scenario is an ultrasound evaluation during the pregnancy – even though the incidence of a malignancy is rare in this case. In peri- or postmenopausal women, the most common scenario would be while performing an evaluation for pelvic–vaginal (PV) bleeding. Since PV bleeding is not a common symptom of ovarian cancer, an adnexal mass found during evaluation for bleeding is an incidental finding [2].
6.1.2 Imaging
Multimodality imaging assessment plays a key role in the management of adnexal masses. Imaging helps to plan the most appropriate therapeutic approach. Since benign ovarian lesions are nowadays treated with laparoscopy, pretreatment imaging findings associated with proper characterization of an ovarian lesion are crucial [5]. The following parameters should be assessed by imaging preoperatively: (a) exact origin of the mass, (b) likelihood of malignancy, and (c), when surgery is needed, the feasibility of laparoscopy versus laparotomy [6].
Transabdominal and transvaginal ultrasonography (TVUS) represents the first-line imaging technique currently used to evaluate ovarian masses [7]. TVUS has been the foremost modality for detection and characterization of ovarian tumors. Especially with the advent of high-frequency transvaginal probes, the quality of images allows description of the lesion’s gross anatomical features, hence making TVUS the method of choice in studying ovarian lesions. This is, however, limited by the great variability of macroscopic characteristics of both benign and malignant masses. Furthermore, the technique is operator dependent. To overcome these limitations, morphological scoring systems have been developed – this being based on specific ultrasound parameters each with several scores according to determined imaging features of the lesion and clinical data. The color Doppler scanning allows the assessment of tumor vascularity. Overall, malignancies display an increased vascularity with decreased peripheral blood flow resistance and increased blood flow velocity compared with benign tissue [2, 8–10]. Nevertheless, a significant proportion of adnexal masses (up to 20 %) remain indeterminate after TVUS.
Magnetic resonance (MR) imaging is now widely accepted as the “problem-solving technique” for the characterization of an indeterminate ovarian mass having both high sensitivity and specificity in discriminating benign versus malignant lesions. Moreover, thanks to its exquisite soft tissue contrast definition, it can address specific diagnosis for certain pathological types [7] (e.g., teratomas, endometriomas).
Computed tomography is commonly performed in preoperative disease burden assessment of a suspected ovarian malignancy [11–13].
6.1.2.1 MRI Assessment of an Adnexal Mass
Patient Preparation and Coil Selection for MRI Assessment
Patients undergoing MRI examination should have fasted for at least 4 h to limit artifacts due to bowel peristalsis. Further reduction of motion artifacts can be achieved with the use of antiperistaltic agents. Imaging is performed with the patient in supine position using a multichannel phase array coil. Endorectal and endovaginal coils have a limited field of view for the assessment of lateral pelvic structures and are therefore not routinely used.
Conventional MRI Sequences
A typical imaging protocol includes T1-weighted images (T1WI) in the axial plane and T2-weighted images (T2WI) in the axial, sagittal, and coronal planes using a small field of view (20 cm), a high resolution matrix (e.g., 256 × 256), and thin section images (3 mm). Fat-saturated T1-weighted images (T1W FS) are used to evaluate the presence of fat or hemorrhage, especially when high signal intensity is seen within the adnexa on T1WI and a dermoid tumor or endometriosis is suspected. In patients undergoing ovarian cancer staging, axial T1WI or fast spin-echo T2WI with a large field of view up to the renal pelvis should be included to evaluate the presence of metastatic disease (e.g., lymph node, liver, or bone metastases).
Contrast-Enhanced MRI Sequences
Gadolinium-enhanced images are useful for the evaluation of complex lesions as they may help differentiating solid components or papillary projections from clots and debris. Multiphase contrast-enhanced MRI is performed by using a three-dimensional gradient echo T1-weighted sequence after administration of 0.1 mmol gadolinium per kg of body weight. Dynamic contrast-enhanced MRI (DCE-MRI) involves repeatedly imaging a predetermined volume of interest every few seconds over 5–10 min during the passage of the contrast agent through the tissue of interest, so that changes in signal intensity can be assessed as a function of time. Semi-quantitative analysis can be performed through several parameters derived from contrast enhancement/time curves.
Diffusion-Weighted MRI (DW-MRI)
DW-MRI is performed using two or more b-values, which include one or more low b-values (50–100 s/mm2) and a high b-value (750–1,000 s/mm2). The apparent diffusion coefficient (ADC) is measured in mm2/s and is calculated by the slope of the line of the natural logarithm of signal intensity versus b-values. The ADC maps are usually displayed as gray-scale images. Areas of restricted diffusion have lower ADC values and appear as a darker shade of gray on the ADC maps compared with areas of freely moving water such as cysts or bladder, which appear as a lighter shade of gray. Areas of restricted diffusion appear bright in the high-b-value images (b-value 750–1,000 s/mm2).
Role of MRI in Characterizing Ovarian Masses
The greatest strength of MRI lies in its ability to characterize the physical and biochemical properties of different tissues (e.g., water, iron, fat, and extravascular blood and its breakdown products) through the use of multiple imaging sequences. There are no MRI signal intensity characteristics that are specific for malignant epithelial tumor, and these tumors should be distinguished based on morphologic criteria. The MRI features most predictive of malignancy are an enhancing solid component or vegetations within a cystic lesion, presence of necrosis within a solid lesion, as well as presence of ascites and peritoneal deposits [14, 15].
6.1.3 Defining the Origin of a Pelvic Mass: Adnexal, Extra-adnexal, and Extraperitoneal
Defining the correct origin of a mass is the first diagnostic step in setting up a treatment strategy. The size, architecture, and location may appear similar in adnexal, extra-adnexal peritoneal masses and even in extraperitoneal lesions. However, special features determining the anatomical relationship of the mass and the surrounding pelvic anatomical structures can help in their differentiation [16]. These parameters include visualization of ovarian structures, the type of contour deformity at the interface between the ovary and the pelvic mass, and the displacement pattern of the vessels, ureters, and other pelvic organs. Particularly in large pelvic lesions, the ovary may often be obscured or totally invaded by the mass [8]. In smaller lesions, when the ovary is not completely obscured, identifying ovarian structures – such as follicles – indicates its ovarian origin. Specific signs such as the “beak” sign and the “embedded organ” sign can aid in better defining the mass relationship with the ovary. When a mass deforms the edge of the ovary into a beak shape, it is likely arising from the ovary. In contrast, dull edges at the interface with the adjacent ovary suggest that the tumor compresses the ovary but does not arise from this [16]. Large ovarian masses typically displace the ureter posteriorly or posterolaterally. However other intraperitoneal lesions (e.g., those originating from the bladder, the uterus, or the bowel) can cause the same pattern of displacement [17]. An adnexal lesion typically displaces iliac vessels laterally. In contrast, extraovarian masses arising from the pelvic wall or lymphadenopathies displace the iliac vessels medially. The mass origin may be further elucidated by tracking the vascular pedicle or the ovarian suspensory ligament [16]. Because of their close anatomical relationship, masses arising from the fallopian tubes often cannot be distinguished from ovarian ones. However, incomplete septa emerging from the wall of a cystic “sausage-like” adnexal mass indicate its fallopian origin [18].
6.1.4 Differentiating Benign from Malignant Lesions
The clinical significance of discriminating benign from malignant masses differs depending on the clinical setting in which the mass is initially detected. For women with symptoms, in whom surgical management may be appropriate whether or not the mass is malignant, the main reason to discriminate between benign and malignant lesions is to set up the correct management. For asymptomatic women, discriminating benign from malignant disease is important both to ensure appropriate management in the setting of malignancy and to avoid unnecessary diagnostic procedures, including surgery.
Several MR imaging findings suggestive of malignancy have been reported in the literature. These can be summarized as follows: complex solid–cystic appearance, presence of wall irregularity and thickening, multiple (>5) septations with nodularity, and vegetations and solid enhancing components after contrast medium administration [19]. Predominantly cystic appearance with thin wall and septa and presence of strong hypointensity on T2-weighted images are more often associated with benign lesions. Secondary features of malignancy representing disease involvement of surrounding/distant structures consist of peritoneal, mesenteric, or omental deposits, pelvic side wall invasion, and lymphadenopathy, although pelvic ascites can also be seen in ovarian torsion, pelvic inflammatory disease, and benign ovarian fibroma [3, 4]. When these criteria are applied, the method sensitivity in detecting malignancy is 91–100 % and the specificity is 91–92 % [19, 20]. More recently, functional MRI techniques such as diffusion-weighted MRI (DW-MRI) and dynamic contrast-enhanced MRI (DCE-MRI) have demonstrated a valid contribution in ovarian masses’ characterization. DW-MRI indirectly informs about tumor cellularity and tumor membrane integrity via apparent diffusion coefficient (ADC) calculation. The ADC value has been reported as useful in discriminating between benign and malignant ovarian surface epithelial tumors [20], although there is still no evidence that DW-MRI can independently characterize an ovarian mass [21–25]. DCE-MRI characterizes the leakage of contrast agent from capillaries into the extravascular extracellular space allowing quantitative measurements that reflect blood flow and vascular permeability. Quantitative DCE-MRI parameters have been shown to correlate to tumor angiogenesis status [26]. Semi-quantitative DCE-MRI parameters and curve analyses have also demonstrated a role in predicting malignancy with an increased specificity compared with conventional MRI [27]. Specifically a relationship between early enhancement of solid tumor components and malignancy has been postulated [5, 28, 29].
The probability of malignancy is also related to the patient’s age. In young women less than 9 years of age, 80 % of ovarian masses are malignant, with the vast majority consisting of germ cell tumors. In women of reproductive age, the overall chance that an ovarian tumor will be malignant is 1 in 15 compared to 1 in 3 by 45 years of age [30].
6.1.4.1 Low-Risk Adnexal Masses
If both pre-assessment probability and imaging studies demonstrate low probability of malignancy, additional tests can be avoided [3].
6.1.4.2 Intermediate-Risk Adnexal Masses
The most challenging decision-making cases are the ones with intermediate risk for malignancy. A large proportion of intermediate-risk adnexal masses represent benign entities. Tests with greater specificity allow point-of-care triage that may obviate the need for surgery that would otherwise be purely for diagnostic purposes. However, in case of malignancy they would potentially offer a more timely diagnosis than a “watch and wait” strategy with interval ultrasound reassessment [3]. Tumor markers such as CA125 may be selectively performed, particularly in the postmenopausal population in which specificity is higher. Alternatively, surgical evaluation can be considered if the perceived risk for malignancy justifies this intervention [3].
6.1.4.3 High-Risk Adnexal Masses
If there is sufficiently high risk, then additional diagnostic studies may not be necessary, and clinicians may wish to proceed with studies most relevant to surgical planning and gyne-oncology referral [3].
6.1.5 Ovarian Tumors by Morphologic Characteristics
Morphological characteristics of adnexal masses provide important information for determining the pathological group of a tumor. Although MR imaging findings are not specific for any particular pathological group, some imaging features are more characteristic of one pathological group than another. Ovarian tumors are usually classified into three groups: cystic masses (unilocular or multilocular), cystic and solid masses, and predominantly solid masses.
6.1.5.1 Cystic Masses
Cystic masses may include nonneoplastic cysts, benign neoplasms, and borderline neoplasms. Functional cysts, para-ovarian cysts, hydrosalpinx, endometriotic cysts, serous cystadenomas, mucinous cystadenomas, and mucinous cystic tumors of borderline malignancy are the ovarian cystic lesions most frequently found.
6.1.5.2 Cystic and Solid Masses
In general, a combined cystic and solid mass strongly supports the diagnosis of ovarian malignancy. Primary epithelial carcinomas and metastatic tumors often show a cystic and solid appearance. Mature cystic teratoma is the important exception to this appearance.
6.1.5.3 Solid Masses
Predominantly solid ovarian masses include benign, borderline, and malignant tumors. The main entities are fibrothecomas, Brenner tumors, granulosa cell tumors, dysgerminomas, epithelial ovarian carcinomas, and metastatic carcinomas.
6.2 Benign Ovarian Lesions
Benign adnexal lesions constitute about 80 % of all ovarian tumors. They mostly occur in young women (<45 years). As for malignant tumors, benign neoplasms arise from one of the three ovarian components: surface epithelium, germ cells, or sex cord–stroma [31].
6.2.1 Physiologic Ovarian Cysts
Ovarian cysts below 3 cm are generally considered physiological. These entities can be divided into functional (associated with hormone production) or nonfunctional [32]. Functional cysts comprise follicular cysts, corpus luteum cysts, and uncommon cysts such as the theca–lutein cysts [33] (Fig. 6.1). Simple cysts are thin-walled (<3 mm), unilocular, and below 3 cm in diameter, but can occasionally grow larger. They usually develop during reproductive age, but may also be found in postmenopausal age and are almost always asymptomatic. The majority are follicular cysts and result from failure of rupture or of regression of the Graafian follicle. They are usually self-limiting and usually regress spontaneously within 2 months. Sometimes they are complicated by rupture and may cause abdominal pain and hemoperitoneum [7]. Most functional cysts appear as unilocular small cystic lesions with high signal intensity on T2-weighted images and intermediate to low signal intensity on T1-weighted images, reflecting simple fluid content. Hemorrhagic cysts usually present high signal on T1-weighted images [10, 34]. The thin wall of functional cysts is best depicted on T2-weighted images as a hypointense structure and on contrast-enhanced images as hypervascular in respect to ovarian stroma [11, 35, 36]. When below 3 cm of diameter, they cannot be differentiated from mature follicles. Unilocular cystadenomas may mimic functional cysts, but a follow-up that shows regression over 2–3 ovarian cycles allow the diagnosis of functional cyst. Corpus luteum cysts may get bigger in size because of internal hemorrhage [2, 37, 38]. Hemorrhagic and corpus luteum cysts usually have thicker walls with relatively higher signal intensity on T1-weighted images and intermediate to high signal intensity on T2-weighted images [11]. Among nonpregnant women, corpus luteum cysts derive from failure of regression or hemorrhage into the corpus luteum [2]. Theca–lutein cysts develop when choriogonadotropin levels are abnormally increased – e.g., in the gestational trophoblastic disease or in the ovarian hyperstimulation syndrome [31, 39]. Progesterone production may persist in corpus luteum cysts, therefore resulting in dysmenorrhea. When these cysts are large in size, they can cause abdominal pain because of rupture, hemorrhage, or torsion. Corpus luteum cysts often require indeed up to 3 months to regress [2, 7]. Both corpus luteum cysts and endometriomas may show hemorrhagic content; however, a prominent T2 shading can be observed only in endometriomas, while corpus luteum cysts contain less concentrated hemoglobin, so the T2 shading is less common [32, 40]. Internal debris and fibrin clots can be differentiated from papillary projections of epithelial tumors by their lack of enhancement [32].
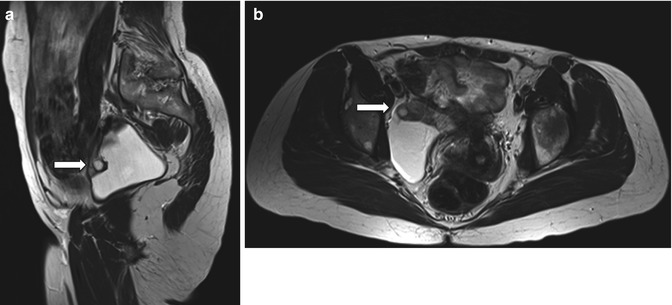

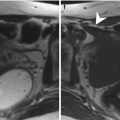
Fig. 6.1
Inclusion cyst. Follow-up scan in a 44-year-old woman with history of endometriosis. Sagittal and axial spin-echo T2-weighted images (TR:4361, TE:80; a, b): right-sided pelvic collection containing the right ovary anteriorly (arrows). There are no suspicious solid components
6.2.2 Endometriomas
External endometriosis is defined as the condition in which endometrial tissue is found in extrauterine sites [40, 41]. Endometrioid cysts, or endometriomas, are benign entities that can reach fairly large dimensions [40]. Malignant transformation is a rare complication (less than 1 %), and the most common histological subtypes include endometrioid and clear cell carcinoma [42].
External endometriosis mainly affects ovaries [41] and bilateral ovarian involvement occurs in 30–50 % of patients [43]. Endometriomas contain an obliterated loculus, lined by endometrial glands [44, 45]. These lesions range from cystic to solid appearance, depending on cyclic modifications under hormonal stimulation – likewise the normal endometrium. As a result, endometriomas may enlarge [7] and their walls can become fibrotic and thickened and may present an irregular external border [46]. These aspects have been explained with the attitude of endometrial loculi to perforate, producing a severe fibrosis as a result of the irritating effect of blood, with subsequent adhesion to the surrounding structures. Another hypothesis is that endometrial cysts have a free exit to the peritoneal cavity and are necessarily sealed off by organized blood or adhesions to surrounding organs [7]. Malignant transformation is a rare complication (less than 1 %); the most common histological subtypes include endometrioid and clear cell carcinoma [42, 47].
On MRI endometriomas usually present as multiple hemorrhagic cysts [34, 48–50]. They have an iron concentration many times higher than even the whole blood [51–53], and this characteristic gives them their typical very high signal intensity on T1-weighted fat-suppressed images and low signal intensity on T2-weighted images [54]. Homogeneous T2 shading, defined as signal loss on T2-weighted images [55], is a characteristic feature of endometriomas, and it mainly depends on their age and the amount of hemosiderin [56]. Other MRI findings include: adhesion to surrounding organs, a distinct low-intensity zone surrounding a cyst loculus on both T1- and T2-weighted images (thick fibrous capsule), loculus content with short T1 and T2 values (recent hemorrhagic content), dependent layering and a T2-hypointense fluid level representing different-aged blood products, and a hypointense peripheral ring caused by hemosiderin staining in chronic lesions [7, 34]. Non-cystic endometriomas may be difficult to identify due to their small size. Nevertheless, these implants are obviously more evident on fat-saturated T1-weighted images and show low signal intensity on T2-weighted images due to fibrosis surrounding glandular islands [57–60]. DW imaging may aid in differentiation between clots and clear cell cancer development within almost-solid endometriomas [48, 60, 61].
6.2.3 Cystadenomas
Cystadenomas are common epithelial ovarian tumors. Two main histological cystadenomas subtypes have been identified: serous and mucinous [33]. Although an overlap exists, imaging features may aid in the differentiation of serous from mucinous cystadenomas [5].
They account for 40–50 % of benign ovarian tumors in the reproductive age, with an increasing frequency with age, so that after menopause cystadenomas account for up to 80 % of the benign ovarian tumors [5, 32]. Cystadenomas are thin-walled unilocular or multilocular cystic lesions filled with serous or mucinous content, sometimes showing hemorrhagic content [22]. Papillary projections within cystic walls are rarely found; therefore, these findings should raise the suspicion of malignancy [22, 62, 63]. Serous and mucinous cystadenomas differ in pathology and prognosis. Serous cystadenomas account for 20–40 % of all benign ovarian neoplasms, with a peak incidence in the 4th to 5th decade of life, and up to 20 % of them are bilateral. They are often unilocular and the inner lining may be smooth or have gross papillary projections [5, 33, 64] (Fig. 6.2). Mucinous cystadenomas account for 20–25 % of benign ovarian neoplasms. They are more common during the 3rd to 5th decade of life and are bilateral in only 2–5 % of cases. They tend to be larger at the time of presentation and are more frequently multilocular, with different contents of the loculi. Mucinous cystadenomas are lined by a single layer of tall, columnar epithelial cells and contain a sticky gelatinous fluid; rupture of a mucinous cystadenoma can result in pseudomyxoma peritonei [5, 22, 25]. At MRI serous cystadenomas have simple fluid signal and hypointense on T1- and hyperintense on T2-weighted images. In contrast, mucinous cystadenomas have often various signal intensities depending on the content of different loculi, varying from simple fluid to proteinaceous to hemorrhagic. Mucin has lower intensity than serum on T2-weighted images. Rarely, mucinous cystadenoma can present as a simple cyst [5].
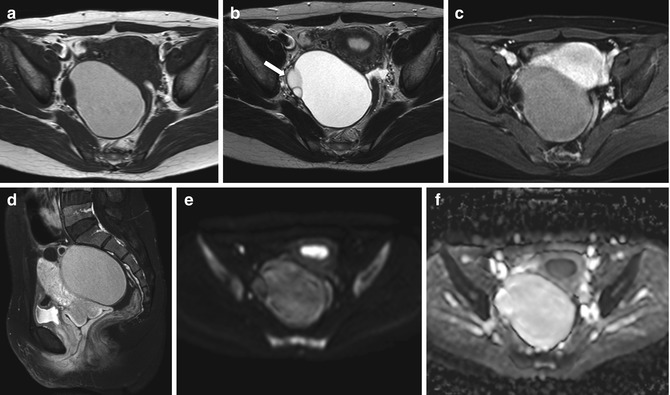

Fig. 6.2
Benign serous cystadenoma. A 32-year-old woman with a right adnexal cystic lesion showing thin septa on the right wall (arrow). The largest component shows high signal on T1-weighted (TR:519, TE:10; a) and T2-weighted imaging (TR:4361, TE:80; b) suggestive of hemorrhagic and/or proteinaceous contents. The remainder of the complex lesion shows low signal on T1-weighted imaging (TR:519, TE:10) and high on T2-weighted imaging (TR:4361, TE:80). There is no significant enhancement following gadolinium (axial and sagittal T1 fat-sat imaging, TR:519, TE:10; c, d). No pathological areas of restricted diffusion (b = 1,000 imaging, e; corresponding ADC map, f)
6.2.4 Adenofibroma and Cystadenofibroma
Adenofibromas and cystadenofibromas are classified as surface epithelial–stromal tumors. They represent about half of all benign ovarian cystic serous tumors and are usually found in women aged 15–65 years [65]. Imaging features are nonspecific and may be similar to malignant or borderline tumors [66]. In fact, the variable amount of fibrous stroma determines various aspects, from oligocystic to complex cystic with solid components or even totally solid, that can mimic ovarian cancer [22, 67]. Almost half of the patients with cystadenofibroma have an abnormal contralateral ovary [68]. Adenofibromas and cystadenofibromas are histologically characterized by fibrous tissue components with epithelial elements, similar to those of ovarian cystadenomas. They can also be completely cystic, with microscopic stromal foci, and their margins are usually well defined and smooth [22, 59, 69]. A black spongelike appearance on T2-weighted images is characteristic of these tumors, consisting of tiny high T2 signal intensity foci within very hypointense solid components, reflecting small cystic glandular structures within a dense fibrous stroma [60]. The majority of solid components shows very low T2 signal intensity and minimal enhancement [5, 6, 33, 60, 70]. Low signal intensity and diffusely or partially thickened cystic wall on T2-weighted images are features suggestive of cystadenofibroma [62] (Fig. 6.3). On DW images, the majority of fibromas and fibrothecomas show both low signal intensity on high-b-value images and low corresponding ADC values (mean 1.156 × 10−3 mm2/s) owing to collagen-producing fibroblasts and a dense network of collagen fibers and also to the T2 blackout effect [24, 25, 60]. Fibrothecomas with degeneration may display intermediate to high signal intensity on high-b-value images [33, 64, 65, 71]. The presence of prominent solid components with high T2 signal intensity and strong enhancement are typical for cystadenocarcinofibroma [62].
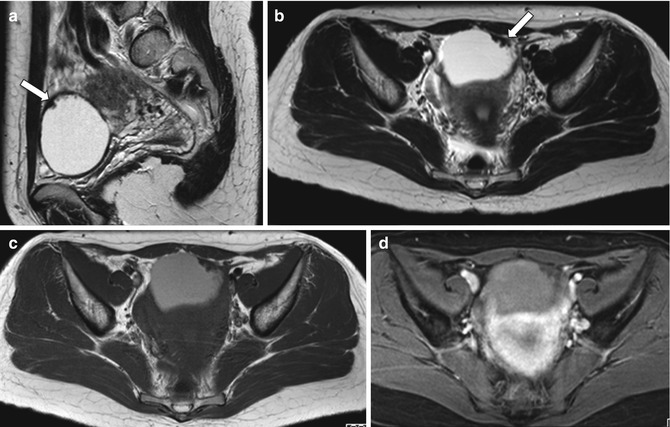

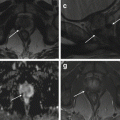
Fig. 6.3
Cystadenofibroma. A 39-year-old woman with normal CA125. Pelvic MRI demonstrates a left-sided complex, predominantly cystic, adnexal mass. The fluid contents are of high signal intensity on T2-weighted (TR:4361, TE:80; a, b) and T1-weighted images (TR:519, TE:10; c) with no signal loss on the fat-sat images (TR:519, TE:10; d) in keeping with proteinaceous/hemorrhagic materials. There is a papillary “carpet-like” wall thickening on the left anterior aspect of the mass with a very low signal intensity on T1- and T2-weighted images (arrows); this demonstrates mild, delayed contrast medium enhancement (post-gadolinium T1-weighted fat-sat image, d)
6.2.5 Brenner Tumor
Brenner tumors are rare (1–3 %), mostly benign and incidentally found solid tumors, belonging to the epithelial surface derived tumors. They are composed of transitional cells with dense stroma, and a malignant component is rare [54]. They represent about 2–3 % of ovarian tumors and usually occur at a mean age of 50 years. They are usually small (<2 cm), solid, and unilateral [54]. Cystic components can be found in Brenner tumors, and in 20–30 % of cases they are associated with mucinous cystadenomas or other epithelial neoplasm [32, 54, 72, 73]. Brenner tumors may rarely produce estrogen; therefore, they can be associated with endometrial thickening [67]. On T2-weighted MR imaging, the dense fibrous stroma presents low signal intensity, similar to that of a fibroma. Extensive amorphous calcification is often present within the solid component [62, 67, 68]. On DW images, Brenner tumors show both low signal intensity on high-b-value images and low corresponding ADC values (mean 1.156 × 10−3 mm2/s) owing to collagen-producing fibroblasts, to a dense network of collagen fibers, and also to the T2 blackout effect [64, 65]. The combination of a multi-septated ovarian tumor with a solid part with extensive calcifications may suggest the presence of a collision tumor consisting of a Brenner tumor and a cystic ovarian neoplasm, like cystadenoma [54].
6.2.6 Dermoid Cyst/Mature Teratoma
These are relatively common benign ovarian lesions, containing structures derived from the three germ cell layers. At imaging, the presence of fat within a cystic ovarian mass is pathognomonic for a mature cystic teratoma [74]. They are the most common ovarian neoplasms below 45 years of age, accounting for up to 70 % of tumors in women below 19 years of age [75]. They are usually unilateral, with only 10–15 % of dermoids found bilaterally [54]. They can be divided into three categories, among which mature cystic teratomas account for 99 %; less common types are the monodermal, which include struma ovarii and carcinoid tumors [54]. Although all three germ cell layers are present, ectodermal components predominate [63]. In the vast majority (88 %), dermoids are unilocular cystic lesions; a protuberance, called Rokitansky nodule or dermoid plug, projects into the cavity and is the hallmark of dermoids. Mature teratomas typically have a lipid content (90 %), consisting of sebaceous fluid, or adipose tissue within the cystic wall or the dermoid plug [73]. The Rokitansky nodule contains a variety of tissues, often including fat and calcifications, which represent teeth (31 %) or abortive bone [54, 74, 76]. A minority (8 %) of dermoid cysts do not demonstrate fat [74]. Dermoids are usually asymptomatic, incidentally discovered, and tend to grow slowly [77, 78]. Malignant degeneration, torsion and rupture, which can cause acute abdomen due to granulomatous peritonitis caused by leakage of the fatty content, can be present [79]; in particular, malignant degeneration, mainly arising from the dermoid plug, occurs in up to 2 % and is usually found in the 6th to 7th decades of life. The risk of malignancy is associated with larger size (>10 cm) and postmenopausal age [80]. Capsule perforation is a sign for malignant transformation of a mature teratoma [81]. At MRI mature teratomas are usually round or oval and sharply delineated; the lipid-laden cyst fluid shows high signal intensity on T1-weighted images and intermediate signal intensity on T2-weighted images (Fig. 6.4). Fat has a high signal intensity on T1- and T2-weighted fast spin-echo images and loss of signal on fat-saturated T1-weighted images. Chemical shift artifacts can be observed, differentiating fat from hemorrhage. There are some typical internal patterns, such as palm tree-like protrusions or dermoid nipples [40, 82, 83]. Calcifications can be missed on MRI due to the low signal intensity on both T1- and T2-weighted images. The presence of sebaceous fluid floating in the peritoneal cavity can suggest rupture [72]. Low ADC values caused by keratin may be useful in differentiation of mature teratomas with little fat from other cystic tumors; despite this, chemical shift imaging seems a better alternative [22, 35, 51]. MR fat-suppressed or chemical shift images are reliable for the differentiation of fat from hemorrhage [84]. In particular, when no or small amounts of fat are present, dermoids cannot be distinguishable from benign cystic ovarian tumors or ovarian cancer [79]. Monodermal teratomas are solid lesions, mainly or exclusively composed of one tissue type. They include struma ovarii, ovarian carcinoid tumors, and tumors with neural differentiation. Monodermal teratomas are typically solid lesions [22]. Struma ovarii is the most common type (3 %); it consists of mature thyroid tissue, but a mixed morphology with acini filled with thyroid colloid, hemorrhage, fibrosis, and necrosis can be found. Rarely, struma ovarii may produce thyrotoxicosis. They present as cystic or multilocular lesions with loculi displaying high signal intensity on T1- and T2-weighted images, without fat [85].
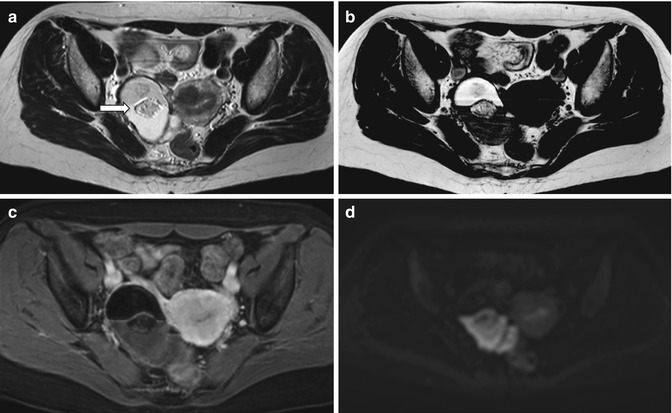

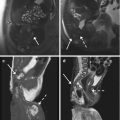
Fig. 6.4
Mature teratoma. A 47-year-old woman with ovarian enlargement on US and normal CA125. Pelvic MRI shows a right-sided complex adnexal mass. There is a fluid–fluid level, as depicted on axial T2-weigted image (TR:4361, TE:80; a) with hyperintense material on the top on axial T1-weighted image (TR:519, TE:10; b), the signal of which is suppressed on fat-saturated T1-weighted pulse sequence (TR:519, TE:10; c) indicative of the presence of fat. There are central areas which are of intermediate signal intensity suggestive of soft tissue (a, arrow) consistent with the typical Rokitansky nodule. These components demonstrate restricted diffusion (hyperintense on the b1000 – d) but do not demonstrate contrast enhancement (c)
6.2.7 Fibroma, Thecoma, and Fibrothecoma
They are sex cord–stromal tumors, composed of spindle cells forming a variable amount of fibrosis and other cells [33] and can occur in both pre- and postmenopausal women. They are solid tumors accounting for 3–4 % of all ovarian tumors, typically unilateral (90 %), occurring in middle-aged and peri- and postmenopausal women. Fibromas are composed of whorled bundles of spindle-shaped fibroblasts with abundant collagen [18, 22, 86]. Fibromas are important from an imaging standpoint because they mimic malignant neoplasms. They can also be associated with ascites and rarely with pleural effusion [87, 88]. The association of an ovarian fibroma, ascites, and pleural effusion is known as Meigs syndrome. Thecomas and fibrothecomas can have estrogenic activity [63]. Bilateral fibromas are also present in basal cell nevus syndrome, along with basal cell carcinomas, bony, ocular, and brain abnormalities as well as other tumors [63].
Fibrothecomas are composed of thecal cells with abundant fibrosis. Unlike fibromas, they can have estrogenic activity and may present with uterine bleeding. In more than 20 %, endometrial carcinomas may be present concomitantly [63]. Because of their abundant collagen content, small fibromas and fibrothecomas have imaging features similar to uterine leiomyomas, with intermediate to low signal intensity on T1-weighted images and hypointensity on T2-weighted images. Larger lesions may have an inhomogenous structure with high signal intensity foci within the low signal intensity lesion, representing edema or cystic degeneration [22, 83, 89] (Fig. 6.5). In larger lesions, dense amorphous calcifications are present but not well appreciated because of their low signal intensity on T2-weighted images. Fibromas and fibrothecomas tend to show mild or delayed gadolinium enhancement [22, 83]. Differential diagnosis should be made with ovarian lesions with fibrous components other than fibroma and fibrothecomas that are cystadenofibroma and Brenner tumor [35, 82]. Pedunculated uterine leiomyomas and broad-ligament leiomyomas can appear as ovarian masses and have the same signal intensity features of fibromas and fibrothecomas. The recognition of the interface vessels between the uterus and adnexal mass is a useful tool in differentiating leiomyomas from ovarian fibromas [90, 91].
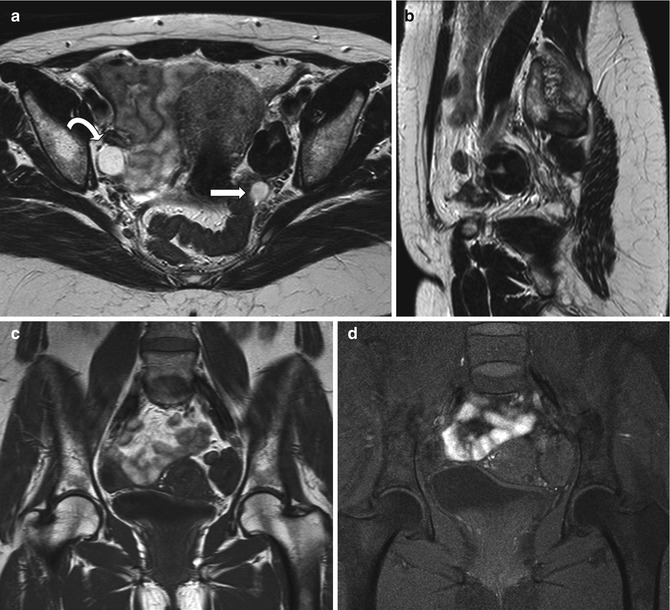

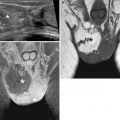
Fig. 6.5
Ovarian fibroma. A 55-year-old lady with pain on the left pelvis. Left ovarian mass showing very low signal intensity on T2-weighted images (axial and sagittal T2-weighted images, TR:4361, TE:80; a, b) and low signal intensity on T1-weighted image (coronal T1-weighted imaging without and with fat saturation, TR:519, TE:10; c, d) in keeping with a fibroma. There is a further small cyst at the posterior margin of the fibroma (arrow). The right ovary shows a small follicular cyst (curved arrow)
6.2.8 Sclerosing Stromal Tumor
Sclerosing stromal tumors are rare benign ovarian tumors occurring in young women [92]. They present as large masses with cystic and solid components. On MRI, they show a mixed hyper- and hypointense appearance on T2-weighted images. On dynamic images, the tumors show early peripheral enhancement with centripetal progression. A central area of prolonged enhancement of the mass represents fibrous hypocellular areas. The appearance after contrast medium administration is useful to differentiate sclerosing stromal tumors from fibromas [85, 93, 94].
6.3 Malignant Neoplasms
6.3.1 Epidemiology and Clinical Features
Worldwide, ovarian cancer accounts for 4 % of all female cancers and is the most frequent cause of death from gynecological malignancy with 239 new cases/year. In the majority of cases, it is a sporadic tumor and presents over the age of 30 years. There is a correlation between age distribution and tumor histology. Epithelial ovarian carcinomas usually present in postmenopausal women, whereas sex cord–stromal tumors and germ cell tumors are prevalent in young women around the age of 20–30 years. Several risk factors for ovarian cancer have been identified: increasing age, nulliparity, early menarche, late menopause, and long-term hormone replacement therapy. There are three recognized hereditary forms of ovarian cancer: breast–ovarian familial cancer syndrome (related to BRCA1 gene mutation), multiple site cancer family syndrome (Lynch II syndrome) and site-specific ovarian cancer. These tumors account for 5–10 % of the total cases; they tend to present mostly in premenopausal women and cause an increased –around 50 % – lifetime risk of developing ovarian carcinoma [95].
The most frequent clinical presentation of all ovarian malignancies consists of abdominal pain and swelling due to the fact that the tumor tends to manifest itself only when it has reached a large size. Functional ovarian neoplasms may present with specific endocrine and non-endocrine syndromes [96]. CA125 is currently the most commonly used serum marker for ovarian cancer, particularly in monitoring treated patients. Its role for initial diagnosis and staging is limited due to low sensitivity and specificity, particularly for stage I ovarian carcinoma. It also has a high false-positive rate in premenopausal women [97].
6.3.2 Staging
The gold standard for staging ovarian cancer remains surgery according to the International Federation of Gynaecology and Obstetrics (FIGO) guidelines. Those are based on the concept that ovarian cancer spreads centrifugally from the pelvis into the peritoneal cavity metastasizing outside the peritoneum only in advanced disease (National Comprehensive Cancer Network – NCCN guidelines for ovarian cancer). A TNM classification has also been defined [98] (Table 6.2).
Table 6.2
TNM staging of ovarian cancer
TNM | FIGO | Imaging findings | Additional findings in surgical/histopathological findings |
|---|---|---|---|
T1 | Stage I | Tumor limited to the ovaries | |
T1a | IA | Limited to one ovary, no ascites | Intact capsule and no tumor on the external surface |
T1b | IB | Limited to both ovaries, no ascites | Intact capsule and no tumor on the external surface |
T1c | IC | Stage IA or IB with ascites | With tumor on surface or capsule ruptured, or ascites or peritoneal washing positive for malignant cells |
T2 | Stage II | Growth involving one or both ovaries, pelvic extension | |
T2a
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|