Fig. 3.1
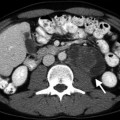
Normal prostate zonal anatomy depicted with T1- and T2-weighted axial MRI at the level of mid-gland. (a) A uniform signal intensity at T1-weighted image where the TZ (arrow) can be hardly differentiated from the PZ (dashed arrow). (b) A high-signal intensity of the PZ (dashed arrow) in contrast with a low-signal intensity of the TZ (arrow) is depicted at T2-weighted image (Courtesy of Ercan Kocakoç, MD, Istanbul, Turkey)
On T2-weighted images, the PZ has a high-signal intensity as compared to the central zone (CZ). The TZ and anterior fibromuscular stroma of IG have low-signal intensity or heterogeneous appearance due to BPH (Fig. 3.1b).
Anatomically, the PZ is surrounded by a true capsule appearing as a thin rim of low-T2-signal intensity. The oval-shaped low-signal-intensity foci surrounded by hyperintense fat on T1-weighted images posterolateral to the capsule represent the neurovascular bundles.
The seminal vesicles normally appear hyperintense and hypointense on T2- and T1-weighted images, respectively.
Prostate-Specific Antigen
Prostate-specific antigen (PSA) is a protein produced by cells of the prostate gland. PSA level can be increased in prostate cancer and benign conditions of prostate such as benign prostate hyperplasia (BPH) and prostatitis. Normal PSA level should be less than 4 ng/mL.
Types of PSA
Three forms of free PSA have been identified in the bloodstream: B-PSA (PSA form produced by BPH tissue), I-PSA (inactivated form of active PSA), and pro-PSA.
B-PSA and I-PSA are reduced relative to normal levels in the blood of prostate cancer patients while the levels of proenzyme (pro-PSA) are increased.
Pro-PSA is better than other forms of PSA to differentiate between cancer and benign conditions in men with PSA values from 2.5 to 10 ng/mL.
PSA Velocity
PSA velocity (PSA-V) defines the rate of change of the PSA level.
PSA-V is helpful in detecting early cancer in men with mildly elevated PSA levels and a normal digital rectal exam.
PSA-V can also be used for predicting the behavior and prognosis of prostate cancer in men undergoing treatment.
A PSA-V of 0.75 ng/mL or greater per year is suggestive of cancer (72 % sensitivity, 95 % specificity).
PSA Density
PSA density (PSAD) is used to correlate PSA and prostate volume.
PSAD is defined as the total serum PSA divided by prostate volume, as determined by transrectal ultrasound measurement. A high PSA density means that a relatively small volume of prostate tissue is making a lot of PSA.
A cutoff value as 0.15 was suggested to improve the detection rate of prostate cancer for patients with PSA levels between 4 and 10 ng/mL. Accurate measurement of prostate volume has critical value in determination of PSA density.
Imaging Findings and Pathological Features
Imaging is crucial for the management of patients with PC. Most of the currently diagnosed PCs are amenable to curative treatment. Importantly, the 5-year survival rate, which is 100 % for the local or regional disease, drops to 34 % if distant metastasis is present. In this regard, increased awareness of the disease as a primary cause of male cancer mortality has generated a new challenge for imaging of the disease. Imaging not only enables the assessment of the stage of the disease but also aids in the early detection of intra- or extraprostatic tumor. Consequently, it guides the selection of various therapeutic options, such as local versus systemic therapy, and is useful for assessing response to therapy. In this regard, primary or recurrent PC can be curatively treated with radical prostatectomy when it is confined to the prostate, whereas chemotherapy, immunotherapy, or hormonal therapies can be used when the tumor extends beyond the gland. Imaging of PC, which is traditionally based on morphological evaluation, is currently being supported by functional imaging techniques.
Measurement of Prostate Volume
Prostate volume can be measured by transrectal or transabdominal ultrasound and MRI. The weight of the prostate is essentially equal to its estimated volume since the specific gravity of the prostate has been calculated to be 1.05.
Currently three techniques are employed to determine prostatic volume: prolate ellipsoid formula, prolate spheroid formula, and planimetric method. Prostate volume is most commonly measured by ellipsoid formula that requires measurement of anteroposterior (length – L), transverse (width – W), and longitudinal (height – H) diameters. Anteroposterior and transverse diameters can be determined in the axial plane while longitudinal diameter can be measured in sagittal plane. Ellipsoid formula of prostate volume is applied as:
Volume = H × W × L × 0.52 (/6).
However, ellipsoid formula is reported to underestimate prostate volume by 7 %–27 % in large prostates.
The prolate spheroid formula W × W × H × 0.52 might be equally accurate as ellipsoid formula and has the advantage of requiring measurements in the transverse plane only.
Step section planimetry is assumed to be the most accurate method of prostate volume determination.
Normal prostate volume ranges between 20 and 30 mL. After 50 years, prostate volume doubles every 10 years, giving a prostate gland volume of more than 40 mL as enlarged in the older man.
Imaging Features
Ultrasound
The evaluation of a suspected PC is the most common indication for transrectal ultrasound (TRUS). Transrectal ultrasound (TRUS) is preferred imaging choice for the evaluation of the PC. Its main role is guide prostate biopsies.
Technique
Biplane transrectal probes with a combination of end-viewing and/or side-viewing wide-band high-frequency transducers allow multiplanar imaging in semicoronal, axial, and sagittal projections.
The patient is instructed to use a rectal enema on the morning of the procedure. A left lateral decubitus patient position is usually preferred as it is well tolerated.
A digital rectal examination (DRE) is performed before probe insertion. TRUS is performed in transverse or semicoronal plane beginning from the level of seminal vesicles above the prostate base, continuing down to the apex. The presence of any asymmetry or suspicious finding detected on axial or coronal imaging is ascertained by scanning from right to left in the sagittal plane. TRUS also enables the measurement of the volume of the prostate.
Gray-Scale Ultrasound
By gray-scale TRUS, PCs appear hypoechoic (60–70 %), isoechoic (40 %), or rarely hyperechoic. However, an inhomogeneous echo pattern may also be seen as the tumor enlarges.
In general PC may appear as a focal nodule, a nodule with an additional infiltrative component or one with a predominantly infiltrative pattern (Fig. 3.2). Not infrequently, an ill-defined lesion may be the predominant ultrasound finding (Fig. 3.3), or the normally isoechoic or hyperechoic echotexture of the PZ may change to a diffusely hypoechoic pattern, which is consistent with advanced PC (Fig. 3.4).
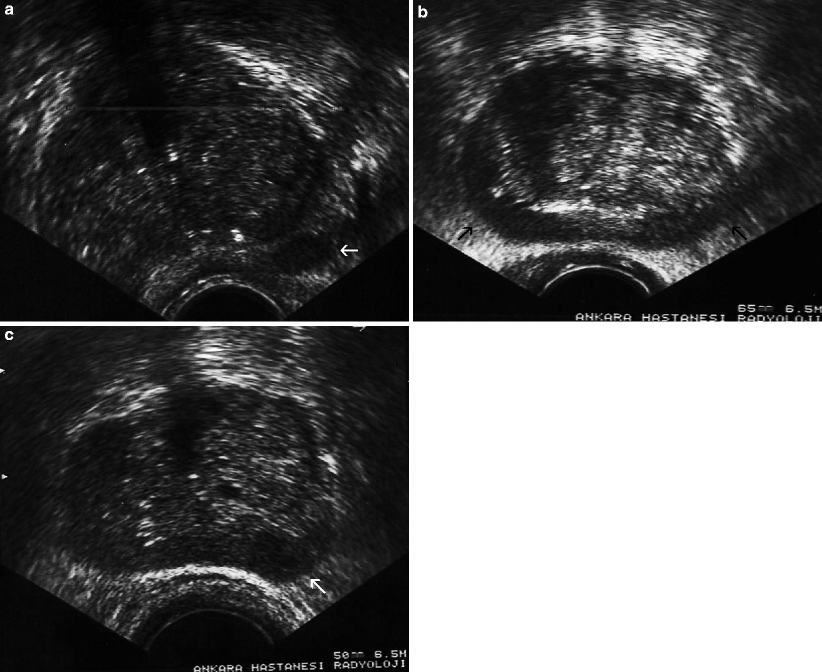
Fig. 3.2
Prostate cancer. Transverse TRUS images demonstrate focal nodular (arrow in a), diffuse infiltrative (arrows in b), and combined nodular and infiltrative (arrow in c) patterns of PC
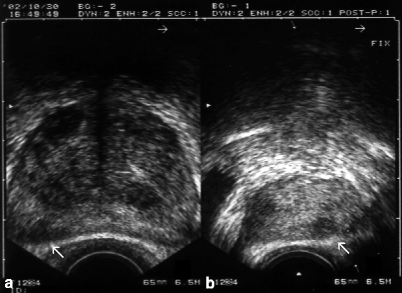
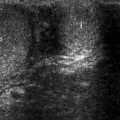
Fig. 3.3
Transverse (a) and longitudinal (b) TRUS scans reveal a small, hypoechoic, ill-defined nodule (arrows) in the posterolateral aspect of the right PZ representing adenocarcinoma of the prostate
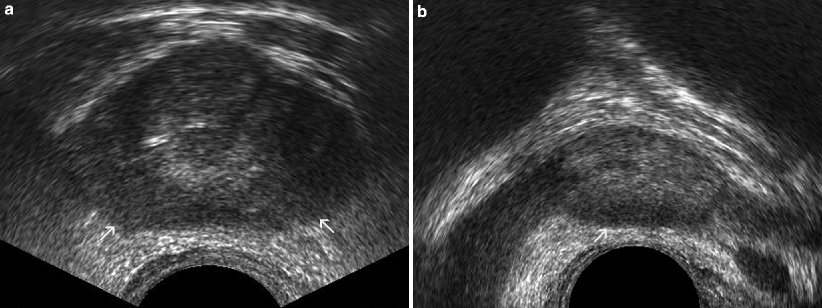
Fig. 3.4
Prostate cancer. Transverse (a) and sagittal (b) TRUS images demonstrate the change of the normally iso- or hyperechoic PZ echotexture with a diffuse hypoechoic pattern (arrows in a and b) which was histopathologically proven to be consistent with adenocarcinoma of the prostate
An asymmetric capsular bulging or irregularity in the normally smooth, well-defined margin along the lateral or dorsal aspect of the prostate without any suspicious parenchymal finding should be regarded as a clue for the presence of PC.
A significant correlation has been detected between Gleason score and echogenicity of the tumoral lesion, with the hypoechoic tumors being moderately or poorly differentiated and isoechoic lesions being well or moderately differentiated.
Sonographically, protuberance and irregularity of the capsule margins, heterogeneous echotexture with hypoechoic strands within the fat planes posterior to the prostate, blunting of the prostate-seminal vesicle junction, and asymmetry of the seminal vesicles are suggestive of extracapsular extension of PC; however, the specificity of this finding is low (Fig. 3.5).
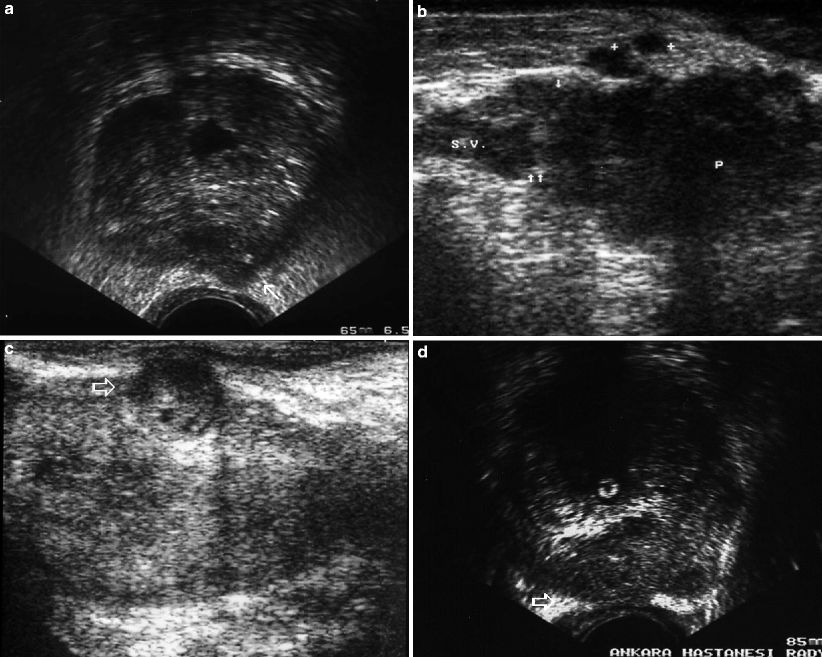
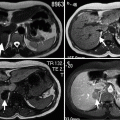
Fig. 3.5
Gray-scale TRUS findings suggesting extracapsular extension of PC. (a) The capsular bulging of a hypoechoic tumor in the left PZ with an apparent thinning and distortion of periprostatic fat (arrows). (b) Tumor with heterogeneous echotexture diffusely infiltrating the prostate with irregular capsule, metastatic periprostatic nodular lesions (plus sign), and loss of seminal vesicle bulging (arrows) (P prostate, SV seminal vesicle). (c) Obvious capsular bulging of a PZ nodule with heterogeneous echotexture (open arrow). (d) A diffusely infiltrative prostate tumor extends into the periprostatic fat and abuts the rectal wall (open arrow) (Courtesy of Eriz Özden, MD, Ankara, Turkey)
In summary, gray-scale TRUS is of limited value for the detection and staging of PC despite being highly accurate for the depiction of prostate anatomy and the assessment of the prostate size. However, it may be useful for monitoring size reduction of hypoechoic lesions after initiation of androgen deprivation therapy.
Color Flow Doppler Ultrasound
It has been suggested that color flow Doppler ultrasound (CDUS) may be helpful for the detection of PC, regardless of the echogenicity of PC. Sonographically, PC is associated with focal, diffuse, and surrounding patterns of blood flow within the lesion, with diffuse flow being the most common (Fig. 3.6).
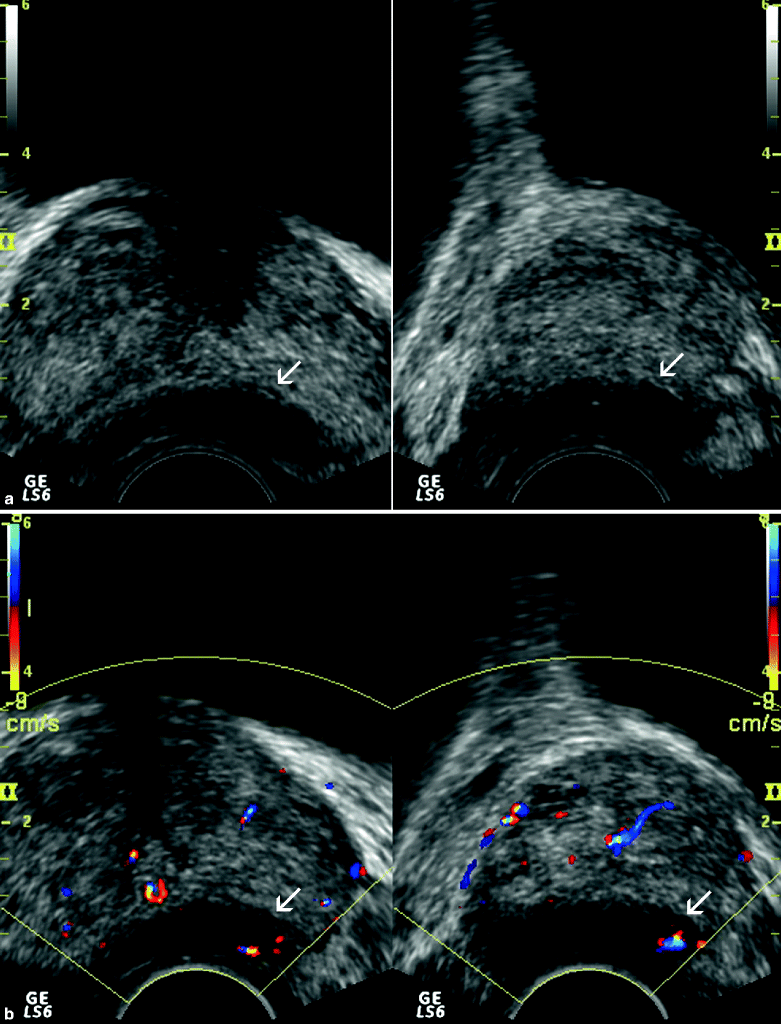
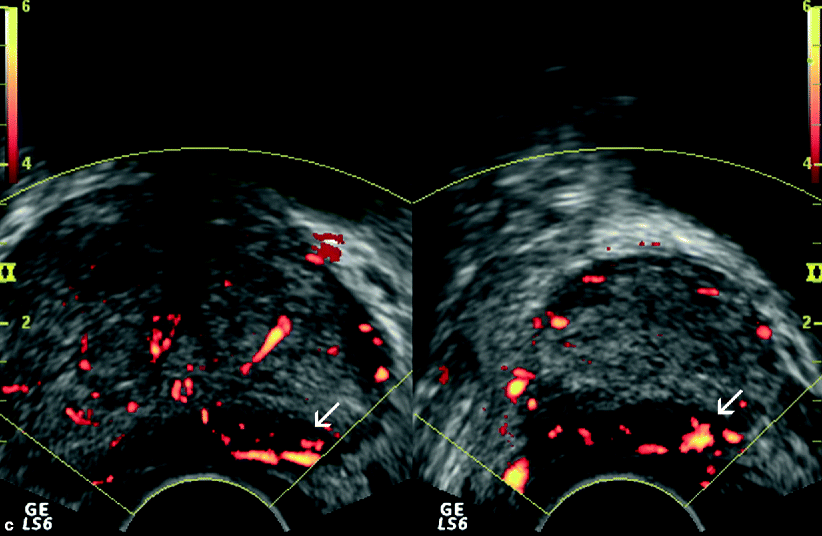
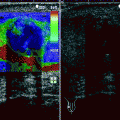
Fig. 3.6
Gray-scale (a), color flow (b), and power Doppler (c) TRUS images obtained at transverse and sagittal planes representing adenocarcinoma of the prostate. (a) A nodular, hypoechoic lesion with peripheral infiltrative component is located at the medial aspect of the base and the mid-gland of the prostate (arrows). (b) A focal type of vascularization is shown within the same lesion (arrows). (c) The vascularization of the lesion is shown to be more extensive by power Doppler TRUS (arrows), which is more sensitive for the detection of the small, low-flow blood vessels
CDUS findings correlate positively with the stage and the grade of PC and with the posttreatment risk of recurrence, in that hypervascularity is associated with higher Gleason scores and implies higher risk of extraprostatic spread. It is generally agreed that the targeted biopsy depending on high-frequency CDUS or power Doppler ultrasound (PDUS) does not preclude the need for systematic biopsies of the prostate.
Power Doppler Ultrasound
PDUS, which is particularly sensitive for the detecting small, low-flow blood vessels, is more useful than CDUS for the diagnosis of PC though it rarely provides any additional benefit (Fig. 3.6).
Spectral index measurements of the capsular and urethral arteries, which are the main feeding arteries of the prostate, may have a role for the detection of PC. Spectral waveform measurements by power Doppler TRUS might enable the differentiation of PC from benign hypertrophy, as mean pulsatility index and resistivity index values are usually lower in the prostate lobes with PC.
There is lack of consensus in published reports regarding the benefit of PDUS for targeted prostate biopsies.
Contrast-Enhanced Ultrasound
Contrast-enhanced TRUS imaging can also be used for the diagnosis of PC (Fig. 3.7). The technique improves the sensitivity for PC detection. Higher degree of enhancement may be associated with higher Gleason scores.
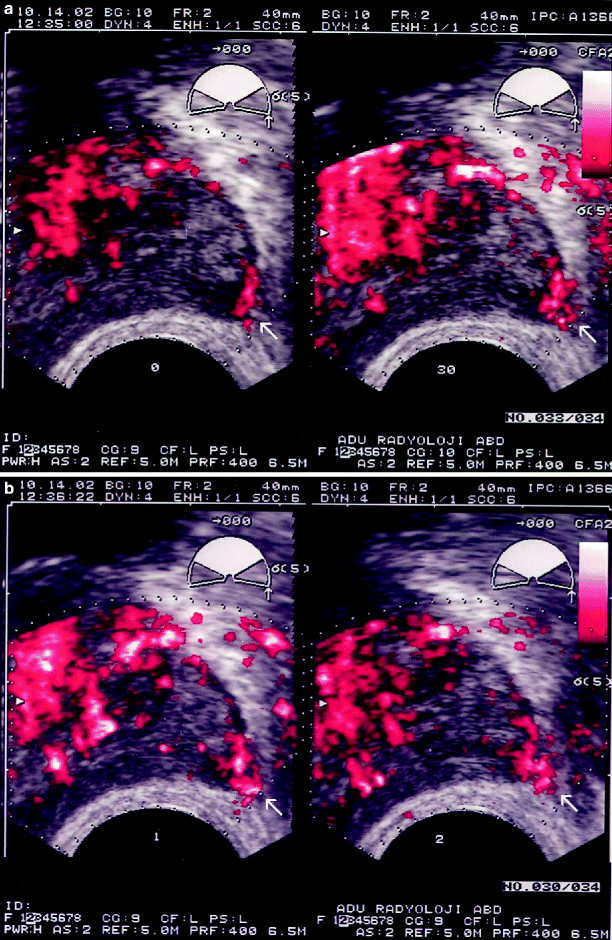
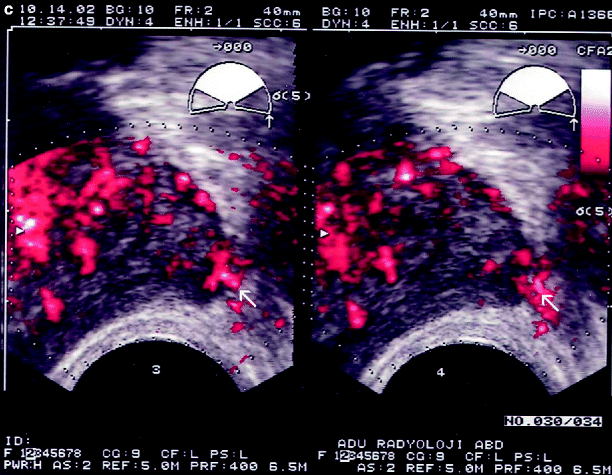
Fig. 3.7
(a–c) Prostate cancer. Peripheral type of hypervascularity in a hypoechoic nodule (arrows) in the PZ detected by power Doppler TRUS has changed into mixed type of hypervascularity suggesting malignancy by consecutive contrast-enhanced power Doppler TRUS scans obtained at 30 s as well as 1, 2, 3, and 4 min after Levovist® injection. The histopathological analysis of the targeted biopsy specimen confirmed adenocarcinoma of the prostate (Courtesy of Can Karaman, MD, Aydın, Turkey)
Targeted biopsies based on contrast-enhanced ultrasound may provide a further benefit of decreasing the number of cores during the biopsy.
Elastography
Elastography can be applied to prostate imaging through a specially designed TRUS probe. During elastography examination, slight compression and decompression is applied on the prostate which changes the real-time ultrasound image constructed.
PC causes an increased cellular density resulting in increased firmness within the gland. The limited elasticity or compressibility of the cancerous prostate tissue is depicted sonographically as dark zones. Decreased tissue elasticity by elastography and abnormal color flow by Doppler US may be correlated with moderate- and high-grade cancers. The incorporation of the elastography to TRUS examination for biopsy guidance has been reported to increase the detectability of PC. Targeted biopsies based on gray-scale ultrasound, CDUS, and elastographic imaging are more likely to yield positive biopsy results compared with systematic biopsies.
Currently, the techniques proposed for use in targeted biopsy are not sufficiently accurate to replace standard systematic biopsy procedures.
Prostate Biopsy
TRUS-guided prostate biopsy is the “gold standard” for the detection of PC. The indications for TRUS-guided prostate biopsies are shown in Table 3.1. Accordingly, abnormal DRE, elevated serum prostate-specific antigen (PSA) levels, and/or suspicious finding on TRUS examination are the indications for TRUS-guided prostate biopsy.
Table 3.1
Indications for TRUS-guided prostate biopsy
Absolute
Abnormal DRE findings
Elevated levels of serum total PSA
PSA velocity >0.75 ng/mL/year
Abnormal TRUS finding(s)
Relative
Free PSA <20 %, total PSA in gray zone
Ratio of pro-PSA to free PSA >1.8 %
Prior to BPH surgery
Prior to salvage local therapy to diagnose and stage prostate cancer recurrence after radiation therapy failure
A PSA level exceeding 4 ng/mL is an indication for TRUS-guided biopsy. Sextant protocol involves obtaining core biopsies at the midway between the lateral border and the median plane at the levels of base, mid-gland, and apex of the prostate, respectively, on each side (Fig. 3.8). However, the use of extended sampling is becoming increasingly common in many centers.
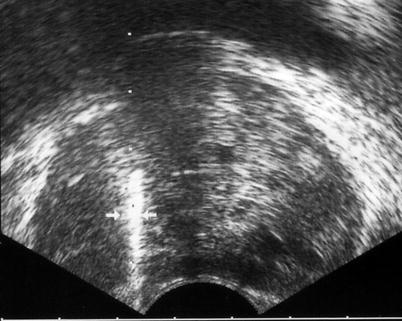
Fig. 3.8
TRUS-guided prostate biopsy. The biopsy needle (arrows) coursing through the mid-parasagittal region of the PZ with the echogenic appearance in the trajectory representing hematoma related to the sampling process
Complications of TRUS-Guided Biopsy
Minor complications can be detected in 70 % of the patients. Minor complications are as follows:
Mild, self-limiting rectal or urethral bleeding may present with hematuria, hemospermia, or rectal bleeding.
Infectious complications: acute prostatitis and urinary tract infections.
Postprocedural pain or discomfort.
Severe complications such as urinary retention and infection, severe rectal bleeding or hematuria, prostate infection, or abscess with fever/sepsis are very rare (less than 1 %).
Computed Tomography
The major role of CT in the management of PC is in the nodal staging of the disease, for which its utility is limited, since microscopic metastatic involvement of lymph nodes cannot be detected by CT or magnetic resonance imaging (MRI). The criterion for regarding a lymph node as being suspicious for metastases is nodal short-axis diameter greater than 1 cm. However, microscopic metastatic involvement of the lymph nodes cannot be detected by CT or magnetic resonance imaging (MRI).
Metastases to solid organs such as the lung, liver, pleura, and adrenal glands can be detected by CT.
Magnetic Resonance Imaging
Conventional Magnetic Resonance Imaging
Fast spin echo imaging, preferably with the combination of endorectal and pelvic-phased array coils, is necessary for an optimal MRI examination of the prostate. However, the use of an endorectal coil at 3.0 T is controversial, as an image with a comparable quality would be obtained, thanks to the improved signal-to-noise ratio provided by the high-field-strength system.
Generally, T1-weighted axial images of the pelvic region are acquired for the detection of nodal involvement and post-biopsy intraglandular hemorrhage. Additionally, thin section (3.0-mm thickness) T2-weighted images in the axial, sagittal, and coronal planes are helpful for the detection, localization, and staging of PC.
Cancer Detection
On T1-weighted images tumors are impossible to discern.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree