12 Neuro IR It is an exciting time for neurointerventionalists. Back in 1995, the NINDS trial demonstrated a clear benefit to the use of intravenous tissue plasminogen activator (tPA) for acute stroke. This remained the standard of care for acute stroke intervention for two decades, until several trials published in 2015 showed improved outcomes with the use of endovascular thrombectomy compared to medical management alone. This has ushered in a new era, with updated treatment algorithms for stroke. Neurointerventional radiologists, interventional neurologists, and neurosurgeons may each have a role in providing these interventions, but this will vary between institutions. With the growth of interventional stroke therapy, IR trainees should aim to develop a good understanding of the diagnosis and triage of patients with acute neurological emergencies. The work-up of a suspected stroke begins before the patient arrives at the hospital. The patient’s history is assessed by first responders using the Cincinnati Prehospital Stroke Scale or the Los Angeles Prehospital Stroke Screen. Higher scores on either scale will prompt first responders to activate the stroke protocol at the receiving hospital. The first decision when the patient arrives in the emergency department is determining if it is truly a stroke. There are many stroke mimics, including encephalitis, seizures, or metabolic disturbances. Typically, the emergency department physician and stroke neurologist work together to make that determination. Patients with sufficient concern for stroke get a noncontrast head CT as soon as they roll into the ED to exclude intracranial hemorrhage. If suspicion is high enough, some institutions also perform a CTA of the head and neck while the patient is still in the CT scanner. Tissue plasminogen activator (tPA) is the first-line standard of care for ischemic stroke patients that meet the inclusion criteria. The NINDS trial (1995) demonstrated the efficacy of tPA in reducing neurologic disability if given within a 3-hour window of symptom onset. The ECASS III trial (2008) expanded upon this by showing the efficacy of tPA given up to 4.5 hours after symptom onset. The current guidelines state that if the patient is an intravenous tPA candidate and symptom onset is less than 4.5 hours, the patient should receive tPA—anything beyond that, tPA is not an option. A number of questions need to be answered as soon as possible to figure out if the patient should get tPA. Initial candidacy is determined by time from last-known-well, symptom severity, and noncontrast head CT findings (▸Table 12.1). Timing of stroke onset can be difficult to determine, as many patients awake from sleep with symptoms. Symptom severity is determined using the National Institutes of Health (NIH) Stroke Scale. CT images are evaluated for presence of hemorrhage at a minimum, and signs of early ischemia if present. After intracranial hemorrhage has been ruled out, other criteria are then reviewed to exclude patients with a prohibitively high risk of complication from tPA. Table 12.1 Inclusion/exclusion criteria for tPA for stroke
12.1 Ischemic Stroke
Approach to a Patient with Suspected Stroke
Management of Ischemic Stroke
Inclusion criteria |
Ischemic stroke |
Symptom onset < 4.5 hours from last-known-well |
Exclusion criteria |
Recent stroke, ICH, head trauma in past 3 months |
CT showing ICH or large, irreversible area of infarct |
Recent head/spine surgery |
Presence of cerebral aneurysm, intracranial neoplasm, AVM |
SBP ≥ 185 or DBP ≥ 110 mm Hg |
Active internal bleeding |
Bleeding diathesis (INR > 1.7, elevated PTT, platelet count < 100,000, etc.) |
Despite being the first-line therapy for acute ischemic stroke, systemic thrombolytic therapy has not been shown to be equally effective in every intracranial vessel. A study from 2010 by Bhatia et al showed that stroke patients with large vessel occlusions had the lowest rates of vessel recanalization following systemic tPA administration, and also the poorest neurological outcomes among all groups studied. This prompted a search for additional techniques to improve outcomes in patients with large vessel occlusions. Advances in mechanical thrombectomy proved to be the solution.
While mechanical thrombectomy has been performed for acute ischemic stroke since the early 2000s, several negative trials precluded its widespread acceptance. MR CLEAN (2015) was the first trial to show the superiority of early mechanical thrombectomy compared to tPA alone in the treatment of proximal anterior large vessel occlusion. It was the largest and most inclusive of five simultaneous trials focusing on thrombectomy. After MR CLEAN was published, all other ongoing trials were stopped early due to overwhelming evidence (ESCAPE in North America, EXTEND-IA in Australia, SWIFT PRIME, and REVASCAT in Spain); however, the data collected from those trials were also strongly supportive. This new evidence has revolutionized the way ischemic stroke is treated.
In situations where tPA is less efficacious, mechanical thrombectomy excels; it benefits patients who have large vessel occlusions, and it is the only intervention potentially available to those who are not tPA candidates. As an added advantage, thrombectomy has a longer window for use in stroke patients.
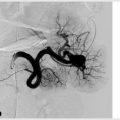
Once the decision has been made to give tPA or not, the next step is to determine if the patient is a candidate for thrombectomy. Three factors are taken into consideration: location of the clot, time from symptom onset, and imaging findings. CTA is usually the go-to for identifying large vessel occlusions, as well as providing a road map for endovascular treatment. Clot in the proximal anterior circulation is most amenable to mechanical thrombectomy. This includes the internal carotid artery (ICA), M1/M2 branches of the middle cerebral artery, and A1/A2 branches of the anterior carotid artery.
Initial guidelines recommended mechanical thrombectomy for an anterior circulation stroke up to 8 hours from the time of symptom onset. In 2018, the DEFUSE 3 trial by Albers et al showed that this window can be extended to as much as 16 hours in select patients. The trial made use of perfusion imaging, which essentially looks for evidence of salvageable ischemic damage (also called penumbra), and differentiates it from irreversible damage. The larger the penumbra, the greater theoretical benefit from intervention. While the earlier trials demonstrated the efficacy of new endovascular techniques and devices, the most recent studies are further guiding decisions by selecting patients who have the highest benefit-to-risk ratio. With the prospect of a longer intervention window, mechanical thrombectomy is only going to take on a more prominent role in stroke therapy, with the potential to create a significant impact on the care of these patients.
The Food and Drug Administration (FDA) has approved three main types of mechanical thrombectomy devices: coil retrievers, aspiration devices, and stent retrievers (“stentrievers”)—the latter two being more common. All three have the same basic function, which is removal of an occlusive thrombus within the artery (Procedure Box 12.1). Complications of these devices include intracerebral hemorrhage, subarachnoid hemorrhage (SAH), distal embolization, dissection, perforation, and non-revascularization.
Patients treated with mechanical thrombectomy are admitted to the ICU for 24 hours following the procedure. Blood pressure is tightly controlled to maintain normo-tension, and serial neuro examinations done to monitor for signs of deterioration. If there is a decline in neurologic function, a head CT is obtained to look for hemorrhagic conversion or cerebral edema.
For all patients, regardless of intervention, risk factors for stroke recurrence or propagation are addressed during the hospitalization. Aspirin is usually started within 24 hours. Medical management for hyperlipidemia and diabetes is optimized. Active smokers are counseled on smoking cessation. An exercise regimen is prescribed to improve overall cardiovascular health. A bedside or fluoroscopic swallow evaluation is done before a diet is prescribed. Physical/occupational therapy and possibly the physical medicine and rehabilitation service should be consulted to begin the rehabilitation process. Finally, screening for atrial fibrillation and carotid disease can be done to assess the risk of embolic events.
Mechanical thrombectomy is a technique used for treating ischemic stroke. The procedure requires two special pieces of equipment: a balloon guide catheter (BGC) and a stent retriever. In order to arrest forward flow through the affected artery, the BGC employs a balloon at its tip; when inflated, the balloon occludes the vessel at a point proximal to the thrombus and prevents distal embolization of thrombus fragments during the procedure by stopping flow.
After obtaining femoral arterial access, the ICA is selected and a diagnostic angiogram performed to identify the site of intracranial vessel occlusion. The BGC is parked proximal to the clot with the balloon deflated. Through the lumen of the BGC, a microwire is deployed and navigated to the site of the thrombus. The occlusion is then crossed by the microwire and microcatheter. The microwire is removed and the microcatheter exchanged for the stent retriever device, deployed at the site of thrombus. The stent is left in place for 5 minutes, which allows the thrombus to incorporate into the interstices of the metallic stent.
The balloon on the BGC is inflated to arrest forward flow. The stent retriever and incorporated thrombus is then retracted into the BGC. While retracting the BGC with stent retriever enclosed, a syringe on the back end is gently aspirated to assist in thrombus removal (▸Fig. 12.1). After removing the stent retriever and trapped thrombus, the BGC is vigorously aspirated to remove any residual components of thrombus in the catheter or treated vessel, as these could embolize and create untreatable distal occlusions.
The balloon on the BGC is then deflated and a diagnostic angiogram is repeated. Because of the manipulation of the vessel, intracranial vasospasm can develop; this is treated with intra-arterial verapamil or nicardipine, and the angiogram is repeated to confirm resolution. Once revascularization has been obtained, strict blood pressure control is essential to decrease the risk of reperfusion hemorrhage.
Fig. 12.1 Photograph of stent retriever shows thrombus which was removed from a patient’s middle cerebral artery. (This image is provided courtesy of Dr Joseph J. Gemmete, MD, University of Michigan Health System.)
For secondary prevention of stroke in patients with noncardioembolic stroke, guidelines recommend the patient take aspirin, clopidogrel, ticagrelor, or a combination drug comprised of aspirin and extended-release dipyridamole. In cases of cardioembolic stroke secondary to atrial fibrillation, an anticoagulation strategy can be determined based on that patient’s CHADS-VASC score.
12.2 Carotid Artery Stenosis
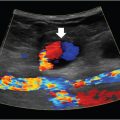
Carotid stenosis secondary to atherosclerotic disease is considered a preventable cause of ischemic stroke, so it’s important to take notice of it, especially in high-risk patients. There’s two different ways carotid stenosis can be discovered: either an asymptomatic patient is diagnosed by screening ultrasound, or neurologic symptoms (transient ischemic attack, syncope, amaurosis fugax, stroke) prompt a search for underlying disease.
Understanding the severity of a patient’s stenosis has become an increasingly complicated task as we gain new knowledge about how to treat these patients. If you’ve learned anything about any of the landmark carotid stenosis trials, you probably remember that the percentage of stenosis is important for stratifying patients. The gold standard for measuring that percentage is angiography, but it is an invasive test and is not routinely used for that measurement alone. Ultrasound, CTA and MRA are noninvasive methods that can be used to approximate the stenosis with relative accuracy. We classify stenosis as mild (< 50%), moderate (50–69%), or severe (≥ 70%).
A number of factors contribute to the strategy for treatment of carotid stenosis including degree of stenosis, presence or absence of symptoms, patient age and comorbidities, as well as an institution’s surgical complication rate. The algorithm for sorting all this out is not as clear-cut as it is for treating stroke, so you should focus instead on understanding the most general guidelines.
Regardless of the severity of carotid stenosis, medical management is the first step. Maximum medical therapy includes both medication (blood glucose control, hypertension, cholesterol, and antiplatelet drugs) as well as lifestyle changes (exercise, smoking cessation, and diet modifications). In select cases, revascularization may be considered in addition to optimal medical therapy. The two options for revascularization are carotid endarterectomy (CEA) and carotid artery stenting (CAS).
Carotid endarterectomy is the gold standard invasive intervention for carotid artery stenosis. For endarterectomy to be an option, the lesion must be accessible (i.e., not too high in the neck). Patients who have undergone previous neck surgery or who have been exposed to previous head and neck radiation are considered higher-risk candidates, and surgery is generally avoided. The patient must also be healthy enough to undergo surgery.
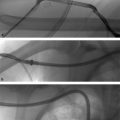
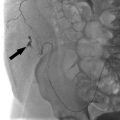
Carotid artery stenting is a minimally invasive endovascular procedure that can be performed by vascular surgeons, interventional cardiologists, interventional neuro-radiologists, and interventional radiologists, depending on the practice setting (Procedure Box 12.2, ▸Fig. 12.2). CAS can be considered as an alternative to CEA in most cases. Some specific indications that favor CAS over CEA include lesions too high for CEA, recurrent stenosis after CEA, treatment of patients with prior head and neck radiation, and those with prohibitively high surgical risk.
Outcomes of major trials also help determine when CAS is appropriate. One of the associated risks inherent to stenting is iatrogenic stroke due to embolizing plaque debris. The development of distal embolic protection devices have helped to minimize this risk. These devices use either a balloon or filter deployed distal to the lesion to be treated, which blocks debris from traveling into the major intracranial vessels.
Choosing between CEA, CAS, or Optimal Medical Therapy Alone
The treatment of asymptomatic carotid stenosis with revascularization over the past 20 years has been guided by the results of a few major trials that took place in the 1990s. The studies showed there was a significant reduction in risk of stroke when CEA was performed for 60% or greater carotid stenosis. However, this finding has been met with skepticism in more recent years. The medical therapy arm of these trials does not reflect the nearly two decades worth of advances in drug efficacy and evidence-based treatment goals.
The goal with CAS is to increase blood flow to the downstream vessels supplied by the stenotic vessel, decrease the risk of plaque fragmentation and thrombosis or embolization, and thereby reduce the risk of stroke.
After obtaining arterial access through the common femoral artery (CFA), an aortogram of the aortic arch is performed. The affected common carotid artery is selected and angiography performed to evaluate vessel diameter, stenosis severity, and downstream perfusion. The stenosis is then crossed with a guidewire, and an appropriately sized embolic protection device (EPD) is deployed distal to the stenosis in a straight segment of the vessel. This will capture embolic debris that may mobilize at the time of stenting.
Predilation of the lesion is performed with a balloon, taking care to monitor for baro-receptor-mediated bradycardia (which may require Valsalva maneuvers or pharmacologic intervention with atropine or glycopyrrolate to reverse). A self-expanding metallic stent is then positioned across the stenosis, deployed, and postdilated. A follow-up angiogram is done to assess residual stenosis and embolic debris burden within the protection device; significant debris within the EPD may require insertion of an aspiration catheter prior to EPD retrieval to prevent the emboli from escaping. The EPD is then retrieved and completion carotid and cerebral angiography performed to confirm revascularization.
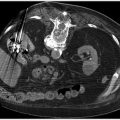
With current optimal medical therapy, some believe that the risk of stroke from carotid stenosis is as low as the periprocedural operative risk associated with revascularization. Others still advocate intervention for asymptomatic patients, but reserve it for those with more severe stenosis, in the 80% or greater range (▸Fig. 12.3).