Tumour stage
Description
1
Localized tumour with complete gross excision, with or without microscopic residual disease; representative ipsilateral lymph nodes negative for tumour microscopically. Nodes attached to and removed with the primary tumour may be positive
2A
Localized tumour with incomplete gross excision; representative ipsilateral nonadherent lymph nodes negative for tumour microscopically
2B
Localized tumour with or without complete gross excision, with ipsilateral nonadherent lymph nodes positive for tumour; enlarged contralateral lymph nodes negative microscopically
3
Unresectable unilateral tumour infiltrating across the midline (beyond the opposite side of the vertebral column) with or without regional lymph node involvement, or midline tumour with bilateral extension via infiltration (unresectable) or lymph node involvement
4
Any primary tumour with dissemination to distant the lymph nodes, bone, bone marrow, liver, skin and/or other organs (except as defined for stage 4S disease)
4 s
Localized primary tumour (as defined for stage 1, 2A or 2B disease) with dissemination limited to the skin, liver and/or bone marrow (limited to infants younger than 1 year, marrow involvement of less 10 % of total nucleated cells and MIBG scan findings negative in the marrow)
Table 13.2
Summary of the International Neuroblastoma Risk Group Staging System (INRGSS)
Tumour stage | Description |
|---|---|
L1 | Localized tumour not involving vital structures, as defined by the list of IDRFs, and confined to one body compartment |
L2 | Local-regional tumour with the presence of one or more IDRFs |
M | Distant metastatic disease (except stage MS tumour) |
MS | Metastatic disease in children younger than 18 months, with metastases confined to the skin, liver and/or bone marrow |
For an accurate evaluation of disease extent, multiple tests are required both at diagnosis and during follow-up, including bone marrow biopsy and urine catecholamine levels as well as anatomic and functional imaging modalities [3, 8].
Metaiodobenzylguanidine (MIBG) structurally resembles the adrenergic neurotransmitter norepinephrine and is taken up by the human norepinephrine transporter (hNET) in the cell membrane of sympathomedullary tissues and is stored into the catecholamine-storing granules and adrenergic nerve endings by the vesicular monoamine transporter (VMAT); once stored in granules, MIBG may be released and taken up again through the same mechanism. The presence of these specific uptake mechanisms (approximately 90 % of NB tumours express hNET) and the prolonged intracellular storage provide the molecular basis for the specific imaging and therapy with radioiodinated MIBG [9, 10]. Functional imaging with 123I-MIBG scintigraphy is considered an essential tool in patients with NB both for initial staging – allowing visualization of the primary tumour and metastatic lesions in the various sites – and response to therapy [3].
This chapter will focus on the role of MIBG scintigraphy for diagnosis and monitoring of NB, with mention to compounds for positron emission tomography (PET) such as fluorine-18-fluorodeoxyglucose (18 F-FDG), fluorine-18-dihydroxyphenylalanine (18 F-DOPA), 68Ga-labelled somatostatin analogues, 11C-hydroxyephedrine (11C-HED) and 124I-MIBG. Also the evolution in the use of 131I-MIBG therapy in high-risk NB will be reviewed.
13.2 Available Diagnostic Tools in Neuroblastoma
Ultrasonography is a widely available, non-invasive and easy to perform imaging technique, which is usually the first imaging modality to be used when an abdominal or pelvic tumour is suspected in a child. US allows accurate localization of the abdominal or pelvic mass, also defining the relationship of the tumour with adjacent organs and vessels [3]. CT and MRI are usually performed for assessing local disease and imaging defined risk factors. The wide availability of multidetector CT machines allows to obtain images very quickly without motion artefacts, so limiting the need for sedation. On the contrary, MRI has long imaging time with need for sedation but has the advantage of higher contrast resolution and the lack of radiation exposure. In any case, the superiority of MRI over CT for local disease assessment has not been demonstrated, and the use of one modality or the other depends on local availability and the radiologist’s preference [3].
Bone marrow involvement is assessed by bilateral bone marrow aspirates and biopsy. Bone scan with 99mTc-diphosphonates was extensively used in the past for the detection of bone metastases. A potential pitfall of bone scan is the high physiologic uptake of the growing metaphysis in children, which may be misinterpreted as metastases or hide small lesions. Currently, bone scintigraphy is usually not required, except in cases with MIBG-negative primary tumour or when MIBG positivity cannot be confirmed, i.e. primary tumour removed before MIBG scan [3].
13.3 MIBG Scintigraphy: Technical Aspects
Procedure guidelines for image acquisition and analysis of MIBG scans in children have been developed, aimed to achieve high-quality studies and a high degree of reliability and reproducibility in interpretation [11–13].
13.3.1 Radiopharmaceutical
Both 131I- or 123I-labelled MIBG are available for diagnostic purposes. In children, 123I-MIBG must be considered the tracer of choice for its lower radiation dose to the patient and superior imaging characteristics: shorter physical half-life (13 h for 123I versus 8 days for 131I), ideal photon energy for gamma camera and single-photon emission computed tomography (SPECT) imaging (159 keV for 123I versus 364 keV for 131I) and lack of beta particle emission [14]. 123I-MIBG is commercially available in Europe since the mid-1990s, while in the USA it has been approved for clinical use in children by the Food and Drug Administration in 2008.
13.3.2 Preparation and Interference
To protect the thyroid from unnecessary radiation dose, thyroid uptake is blocked by the administration of saturated solution of potassium iodide administered orally starting 1 day before 123I-MIBG administration and continuing for the duration of the scanning period. Dosage is chosen in accordance to local protocols or European guidelines [11, 15]. In our centre we have adopted the following protocol: 2 mg/Kg per day of potassium iodide (Lugol’s solution 5 %: 1 mL = 130 mg iodide; 1 gtt = 6.5 mg iodide), beginning 1 day before tracer injection and continuing for 1–2 days. Alternatively, potassium perchlorate may be given at a dose of 8 mg/Kg three times daily, starting 2–24 h before tracer injection and continuing for 2 days [13].
Many classes of drugs are known or are expected to alter MIBG uptake and/or vesicular storage through various mechanisms and must be withdrawn before imaging to avoid false-negative results (Table 13.3) [16]. The most commonly used in children are the decongestant pseudoephedrine (a cold and cough preparation) and the beta-adrenergic antagonist labetalol (for blood pressure control) [13]. Sedation may be required due to the long scanning time, mainly in children between 1 and 3 years [13].
Table 13.3
Drugs known to interfere with MIBG
Mechanism of interference | Drugs | Suggested period of withdrawal (days) |
|---|---|---|
Inhibition of uptake-1 | Cocaine, opioids | 7–14 |
Tricyclic antidepressants (amitriptyline and derivatives, imipramine and derivatives, amoxapine, loxapine, doxepin and others) | 7–21 | |
Antipsychotics (phenothiazinesa, thioxanthenes, butyrophenones) | 21–28 | |
Labetalol, metoprolol | 21 | |
Inhibition of granular uptake | Reserpine, tetrabenazine, etc. | 14 |
Competition for granular uptake | Norepinephrine, serotonin, guanethidine, etc. | 14 |
Depletion of storage granules | Reserpine, guanethidine, labetalol, etc. | 14–21 |
Sympathomimeticsb (such as phenylpropanol-amine, amphetamine, dopamine, isoproterenol, salbutamol, etc.) | 14–21 | |
Increased uptake and retention | Calcium channel blockers | 14 |
Angiotensin-converting enzyme inhibitors | 14 |
13.3.3 Administered Activity
Some differences exist in the choice of administered activity of 123I-MIBG in children between European and North American guidelines. The North American Consensus Guidelines recommend administered 123I-MIBG activities of 0.14 mCi/kg (5.2 MBq/kg) with a minimum of 1 mCi (37 MBq) and a maximum of 10 mCi (370 MBq) [17]. In the paediatric dosage card by the European Association of Nuclear Medicine (EANM), the administered activity is calculated by multiplying a baseline activity by different multiples (weight- and radiopharmaceutical-dependent factors) with a minimum activity of 37 MBq and a maximum activity of 400 MBq for 123I-MIBG (EANM Dosage Card, Version 1.2.2014, www.eanm.org). In any case, as sensitivity of MIBG scintigraphy increases with increased administered activities and the risk of radiation hazard is less than the risk deriving from false-negative results, it is of great importance to get high-quality images with adequate counts [13]. To avoid adverse effects (tachycardia, pallor, vomiting, abdominal pain), slow intravenous injection over the course of 1–5 min is recommended. Central venous catheters should be avoided if possible; if these are utilized, they should be flushed with saline solution (100–200 ml), to avoid artefacts in scintigraphic images. The radiation burden depends on administered activity and on the child’s age; the effective dose is 0.037 mSv/MBq for a 5-year-old child and 0.068 mSv/MBq for a 1-year-old child [15].
13.3.4 Instrument Specifications
When 123I-MIBG is used, imaging is performed using a dual-head large-field-of-view gamma camera equipped with a low-energy high-resolution collimator, energy setting 159 keV, 15–20 % window. According to some authors, medium-energy collimation is preferable for acquisition of both planar and SPECT 123I-MIBG images with the aim of minimizing scatter [8]. The availability of SPECT/CT hybrid systems equipped with high-resolution CT is currently growing; co-registered CT images are utilized for attenuation correction and lesion localization.
13.3.5 Imaging Procedure
Standard imaging (planar and SPECT or SPECT/CT) is performed at 20–24 h after 123I-MIBG injection. Additional planar images can be performed at 48 h to clarify tracer accumulation in the kidneys or in the bowel. Anterior and posterior whole-body images are acquired at a scan speed of 5 cm/min, with additional spot images including lateral views of the skull; in alternative, both anterior and posterior static spot views (about 500 kcounts or 10-min acquisition, 256 × 256 matrix; for the upper and lower limbs, 75–100 kcounts may be sufficient) of the head (both anteroposterior and lateral views are recommended), neck, chest, abdomen, pelvis and upper and lower extremities. Planar images do not require computer analysis.
Single-photon emission tomography (SPECT) can improve diagnostic accuracy, allowing better definition and localization of tumour deposits as well as the distinction between physiologic and abnormal uptakes [18]. SPECT is particularly useful when uncertainty exists regarding lesion localization, for example, in distinguishing between soft-tissue and skeletal lesions. Whenever possible, SPECT should be performed even though sedation may be required. Acquisition parameters for SPECT imaging depend on the available equipment; generally 360° rotation, 120 projections, 25–35 s per step and 128 × 128 matrix are used. Ideally, SPECT should cover the thorax, abdomen and pelvis. In SPECT/CT imaging the CT scan should be acquired with high resolution in order to provide better anatomical details. Also reconstruction parameters for SPECT depend on the equipment and software available.
13.4 MIBG Scintigraphy: Clinical Information
13.4.1 Principal Information, Pitfalls, Limitations
Knowledge of normal biodistribution of radioiodinated MIBG is essential to avoid misinterpretation [19]. Normal scintigraphic pattern in children includes visualization of the salivary glands, heart, liver, adrenals and urinary collecting system. The myocardial uptake may be particularly intense in children under 6 months [19]. Tracer accumulation in the skeletal muscles, nasal mucosa, lungs, bowel and thyroid may also be seen. In children, bilateral symmetrical activity is sometimes evident in the neck and supraclavicular region, related to uptake in brown adipose tissue, which is mediated by the sympathetic nervous system [20]. No skeletal uptake is ever evident; the spine is represented by a vertical photopenic strip, and the joints are seen as photon-deficient areas surrounded by background muscle activity (Fig. 13.1a, b).
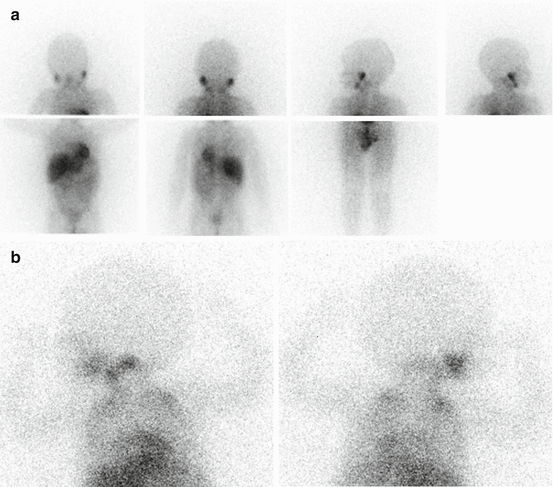
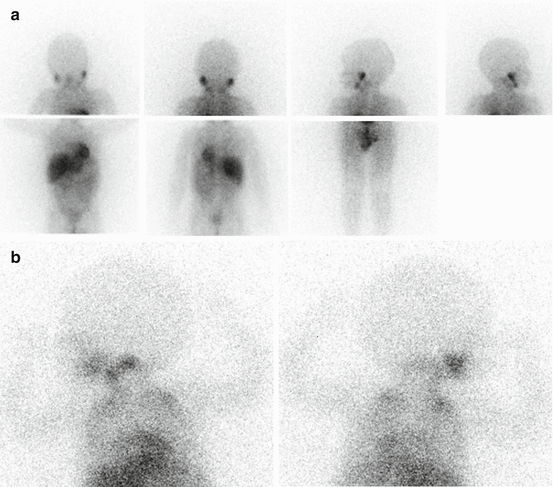
Fig. 13.1
(a) Normal biodistribution of 123I-MIBG. Note the vertical photopenic strip representing the spine and the joints seen as photon-deficient areas surrounded by background muscle activity. (b) 123I-MIBG left lateral and right lateral spot views of the head-neck and thorax. Note the bilateral symmetrical activity in the supraclavicular region related to uptake in brown adipose tissue
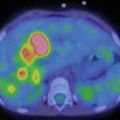
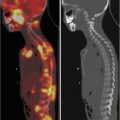
In NB patients, MIBG uptake is observed in the primary tumour and in metastatic sites including the lymph nodes, liver, bone and bone marrow. Skeletal abnormalities can be observed either as focal areas of increased uptake, mostly reported as cortical bone metastases (Fig. 13.2), or as a diffuse uptake, mostly reported as bone marrow infiltration (Fig. 13.3). Recently, the existence of two MIBG-avid metastatic patterns in newly diagnosed NB has been described: a “limited and focal” pattern, found mainly in patients with MYCN-amplified NB, and an “extensive and diffuse pattern” found mainly in patients with single-copy MYCN tumours; in patients with MYCN-amplified NB, focal metastases had a better prognosis than the other types of metastases [21].
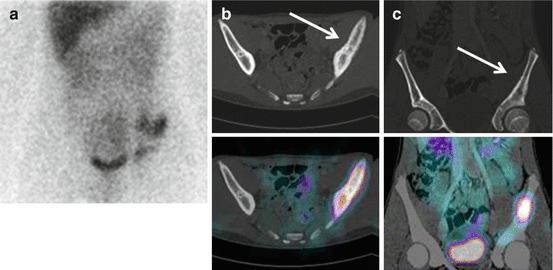
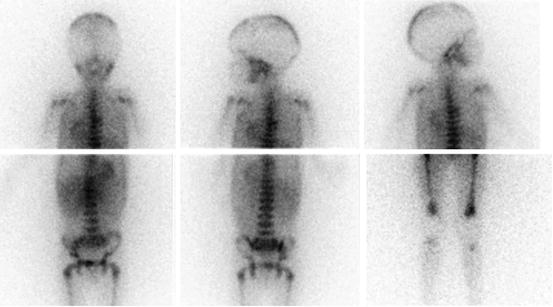

Fig. 13.2
A 13-year-old boy with recurrent neuroblastoma. (a) 123I-MIBG anterior spot view of the abdomen and pelvis showing a focal area of increased uptake in the left region of pelvic bone. Axial (b) and coronal (c) 123I-MIBG SPECT/CT images. Note the cortical bone abnormality evident in the CT images (arrow) and the corresponding area of increased tracer uptake at the fused images

Fig. 13.3
A 4-year-old boy with stage 4 neuroblastoma who previously undergone right adrenalectomy. Note the diffuse skeletal uptake at the level of the skull, humeri, thoracic cage, spine, pelvis, femora and tibia, related to extensive bone marrow involvement
False-positive results at MIBG scintigraphy are an uncommon finding, mainly related to misinterpretation of physiologic uptake, i.e. as a result of radioactivity in the urinary tract, or the presence of adrenal hyperplasia after contralateral adrenalectomy [16]. Moreover, mature ganglioneuroma and other neuroendocrine tumours may show MIBG uptake. SPECT images of the liver should be interpreted with caution, due to heterogeneity of liver uptake; a relatively higher MIBG uptake in the left liver lobe has been reported [22]. The risk of false-positive findings due to misinterpretation of physiologic uptake is expected to be reduced by a more widespread use of SPECT/CT.
False-negative findings at MIBG scintigraphy may be encountered, which are caused by various factors [16]:
Incorrect patient preparation, with reduced MIBG uptake by interfering drugs
Technical factors, such as limited spatial resolution, with reduced sensitivity in detecting small lesions
Anatomical factors, such as tumour size and site (voluminous primary tumours or physiologic uptake masking adjacent small tumour lesions)
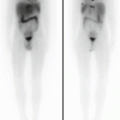
Biological factors depending on intrinsic tumour characteristics, such as tumour heterogeneity, low or absent expression of hNET or rapid tracer washout from the storage pool (Fig. 13.4)
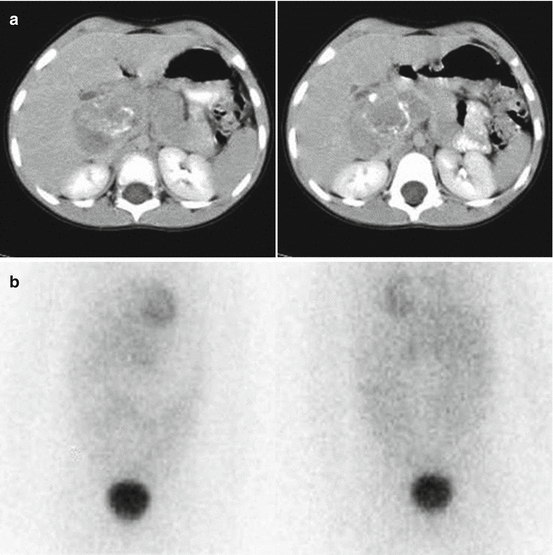
Fig. 13.4
A 5-year-old girl with a voluminous abdominal neuroblastoma evident at two representative axial-enhanced CT images (a) and showing no 123I-MIBG uptake (anterior and posterior spot view of the lower chest, abdomen and pelvis) (b)
13.4.2 Added Value and Clinical Indications
MIBG scintigraphy is a well-established procedure in the diagnostic management of NB allowing visualization of primary and residual/recurrent tumours as well as metastatic lesions in the bone, bone marrow, lymph nodes and other sites with an overall accuracy of about 90 % and a detection rate for bone lesions of about 95 % [16]. Since 1993 the INSS has adopted MIBG scintigraphy for initial staging and response evaluation after therapy [6]. According to the recent INRG Staging System, 123I-MIBG scintigraphy is mandatory at staging; MIBG findings unequivocal for metastatic disease do not require confirmation by other imaging modalities [7]. The specificity of MIBG is close to 100 %. In assessing response to therapy, MIBG scintigraphy is a very sensitive indicator of residual active tumour. Performed at initial diagnosis and after induction chemotherapy, it gives prognostic information, as a positive scan during and after induction therapy or immediately before myeloablative therapy suggests a poor outcome (Fig. 13.5a, b) [23, 24].
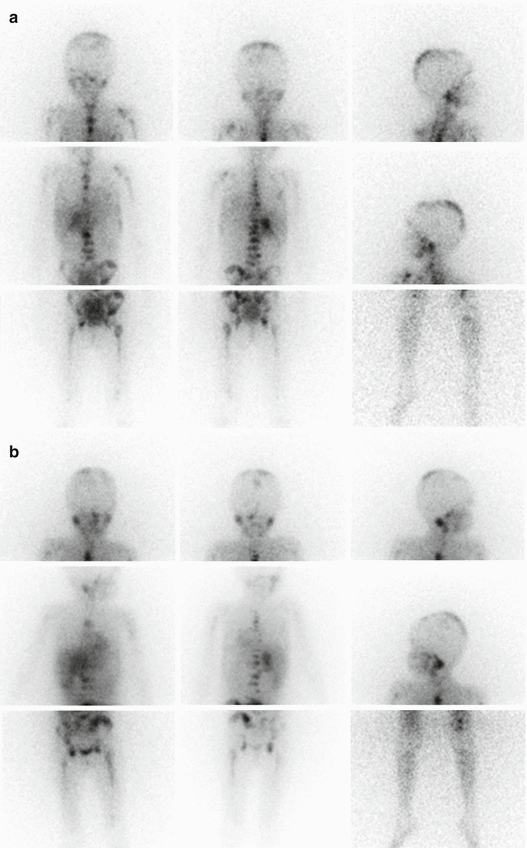

Fig. 13.5
(a) Planar spot views of 123I-MIBG scan in a 5-year-old girl with stage 4 neuroblastoma MYCN amplified. The staging scan shows abnormal 123I-MIBG uptake in the right suprarenal region and extensive bone involvement at the level of the skull, humeri, thoracic cage, spine, pelvis, femora and left tibia. (b) 123I-MIBG scan performed to monitor the response to induction chemotherapy showing persistent abnormal 123I-MIBG uptake in the right suprarenal region and extensive bone involvement
On the whole, MIBG scintigraphy is considered the most effective indicator of NB with the following clinical indications:
Staging of the disease at initial diagnosis
Search of postsurgical residual tumours
Evaluation of tumour response to treatment
Early diagnosis of recurrence at follow-up in high-risk patients
As a prelude to 131I-MIBG therapy
13.4.3 Criteria for Evaluation of Disease Extent
To evaluate the prognostic significance of disease extent at diagnosis and to estimate the individual tumour burden of metastatic disease after therapy, various semi-quantitative scoring systems have been developed for MIBG scintigraphy [25–28]. These scoring systems, which are quite similar with minor variations, aim to predict the extent and the severity of MIBG-active disease as well as improve the concordance between readers of MIBG scan [13, 29]. The body is divided into anatomical regions (from 7 to 12 in the various systems); an individual score for extension of metastatic involvement and intensity of uptake is assigned to each region. Soft-tissue metastases may or may not be included in these scores. The validated semi-quantitative scoring methods that are currently used have been reviewed, with recommendations for their use in response assessment and prognostic evaluation [13, 29]. The Curie scoring system has been validated in France and is now widely used by the Children’s Oncology Group and the New Approach to Neuroblastoma Consortium in the USA [8, 13]. The recently developed SIOPEN score is the one currently used in Europe [13]. A significant prognostic impact of the initial MIBG score and the pattern of MIBG uptake after chemotherapy in patients with stage 4 NB have been recently demonstrated, as higher MIBG scores at diagnosis and the presence of any residual MIBG-positive metastasis after chemotherapy are predictive of unfavourable outcome [30].
13.5 PET Radiopharmaceuticals for NB
In recent years, the functional imaging of NB has been enriched with the use of various radiopharmaceuticals for positron emission tomography (PET). The major advantages of PET imaging versus SPECT are the improved spatial resolution and the shorter time of imaging (single acquisition on 1-day session). From a technical point of view, the standard use of hybrid machines such as PET/computed tomography (CT) allows to routinely correlate anatomic and functional information, thus improving diagnostic accuracy.
A number of studies have confirmed the possibility of depicting NB lesions by PET and PET/CT with 18 F-FDG. A weight-based activity per kg according to the EANM (Version 1.2.2014, www.eanm.org) Dosage Card is administered. As FDG uptake reflects glucose metabolism by cancer cells, FDG PET/CT is expected to be useful for assessing those tumours which fail to accumulate MIBG due to reduced expression of catecholamine transporters or pharmacological interference. However, 18 F-FDG is less specific than MIBG, being concentrated in many tumour types, including benign fibro-osseous lesions as well as in sites of infection/inflammation [31]. The high physiologic brain activity can limit interpretation of 18 F-FDG PET images by masking small skull lesions. Another limiting factor is the physiologic bone marrow activity during chemotherapy or under granulocyte colony-stimulating factor, which can make bone marrow involvement after chemotherapy difficult to visualize. When comparing MIBG scintigraphy with 18 F-FDG PET or PET/CT in NB, MIBG appears to better depict disease extension in stage 4 NB with metastatic involvement of the bone and bone marrow, while 18 F-FDG can be especially useful for detecting soft-tissue sites of disease in the chest, abdomen and pelvis due to the higher spatial resolution of PET technique [32, 33]. In clinical practice, the use of 18 F-FDG PET/CT as a substitute of MIBG scintigraphy is currently not justified. However, in selected cases 18 F-FDG PET/CT may be useful as a complementary imaging modality (Fig. 13.6). The main advantage of 18 F-FDG PET/CT is in those cases that show faint or no MIBG uptake in NB lesions or in case of discrepancies between morphologic imaging modalities and MIBG scintigraphy [8, 34, 35]. Besides disease detection, the results of 18 F-FDG PET/CT have prognostic significance in high-risk NB, as intense metabolic activity in tumour lesions exceeding the tumour avidity for MIBG is associated with more aggressive disease and poor outcome [35].
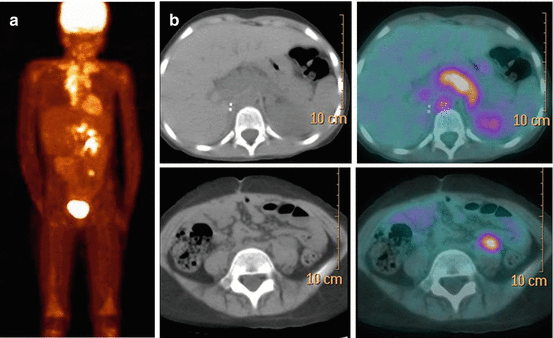

Fig. 13.6
The same patient as in Fig. 13.4. 18 F-FDG PET/CT (a, b) performed at disease recurrence. (a) MIP (maximum intensity projection) image and (b) axial CT and fused images of 18 F-FDG PET/CT, showing abnormal tracer uptake in the chest, upper and lower abdomen and bone corresponding to sites of disease recurrence
New radiopharmaceuticals for PET imaging, which reflect different metabolic pathways of NB cells, such as the uptake of hormone precursors (18 F-DOPA), the expression of receptors (68Ga-labelled somatostatin analogues) or catecholamine metabolism (124I-MIBG, 11C-HED), are currently under investigation.
18 F-DOPA PET/CT, which is considered a valuable tool in patients with pheochromocytoma/paraganglioma and medullary thyroid carcinoma [36, 37], has been recently proved to be a promising tool also in NB patients, for detecting relapse and assessing the response to induction therapy [38, 39]. Administered activity is 4 MBq/kg [40]. When compared with 123I-MIBG scintigraphy, 18 F-DOPA PET/CT seems to be more accurate in depicting primary tumours as well as small MIBG-negative metastases [40, 41]. The predictive role of 18 F-DOPA PET/CT at the time of suspected NB relapse has been recently investigated [42]. By applying an appropriate scoring system, the imaging scores proved to be related to patient outcome in terms of progression-free survival and overall survival, with a significant positive correlation between 18 F-DOPA PET/CT and MIBG scan. Prospective studies comparing these two imaging modalities at initial staging are required to assess diagnostic accuracy and clinical impact of 18 F-DOPA PET/CT in NB.
Like other neuroendocrine tumours, NB is characterized by over-expression of somatostatin receptors, mainly the subtype 2 [43]. In the past, somatostatin receptor scintigraphy has been reported to visualize tumour sites in patients with NB with lower sensitivity than MIBG scan (64 % versus 94 %) [44]. Nevertheless, 111In-pentetreotide scintigraphy can provide prognostic information as a longer survival has been reported in patients with somatostatin receptor-positive NB [43, 44]. Recently, the rationale of the use of radiolabelled somatostatin analogues in NB has been applied to PET radiopharmaceuticals. Preliminary data with 68Ga-peptides report a high sensitivity of 68Ga-DOTATOC PET/CT in NB, >95 % [45]. Furthermore, important therapeutic implications are inherent to the use of 68Ga-peptides in NB, as a positive scan allows to select children who are candidates for radio-receptor therapy with 90Y- or 177Lu-labelled somatostatin analogues [45, 46].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree