CHAPTER 44 Neurotoxicity Associated with Pediatric Malignancies
Cancer is the most common cause of death in children up to 14 years of age; nearly 1 child in 600 will develop cancer. By 2010, 1 in 540 adults who are 20 to 34 years of age is predicted to be a survivor of childhood cancer with variable neurologic sequelae. The neurologic sequelae resulting from the therapy for childhood malignancy vary with the age at presentation, the site of origin of the malignancy, and the therapy. The most common pediatric malignancies associated with adverse neurologic affects are acute lymphoblastic leukemia (ALL), which accounts for approximately 25% of childhood malignancies, and brain tumors, accounting for about 20% of pediatric malignancies. The iatrogenic neurotoxicities may be due to chemotherapy—most often methotrexate (MTX), cranial irradiation, or bone marrow transplantation (BMT). The treatment for ALL involves multiple chemotherapeutic agents, the mainstay of which is MTX. Given intravenously, MTX crosses the blood-brain barrier, eradicating leukemic cells in the central nervous system (CNS), a common site of leukemic recurrence. MTX is also given sequentially via direct intrathecal injection to eliminate leukemic cells from the cerebrospinal fluid (CSF). Another chemotherapeutic agent used in ALL is the enzyme L-asparaginase, which is also associated with neurotoxicity but with a lower incidence and different pathophysiology and imaging abnormalities. Craniospinal irradiation is reserved for ALL patients with CNS relapse or for patients who cannot tolerate high-dose MTX; the doses given range from 1200 to 1800 cGy, which is considerably lower than that used for control of primary CNS malignancies.
CHEMOTHERAPY-INDUCED NEUROTOXICITY
This category includes MTX leukoencephalopathy and microangiopathic leukoencephalopathy.
Epidemiology
MTX neurotoxicity is characterized as acute, subacute, or chronic.1,2 The incidence of acute MTX neurotoxicity ranges from 3% to 10% and varies with the dose and route of administration of MTX, the frequency with which MTX is given, and the use of leucovorin. The time from induction to the onset of acute neurotoxicity varies from 2 to 127 weeks. However, the temporal relationship between acute neurotoxicity and intrathecal administration of MTX is fairly predictable, because acute neurotoxicity is most often seen 10 to 11 days after an intrathecal dose of MTX has been administered.3 High doses of intravenous MTX are also associated with white matter changes on MRI, which may be clinically occult. Subacute neurotoxicity is most often seen 6 to 11 days after intrathecal therapy, whereas chronic or delayed MTX neurotoxicity is seen months after the initiation of therapy.
Clinical Presentation
Acute MTX toxicity occurs within hours to days after administration of MTX and consists of nausea, vomiting, headache, somnolence, altered mental status, and seizures.1,2 Subacute neurotoxicity consists of seizures, focal motor or sensory deficits, behavioral changes, and aphasia; the neurologic deficits usually resolve within 72 hours. Chronic or delayed MTX neurotoxicity varies from a mild increase in distractibility to severe encephalopathy. Consistent long-term deficits seen in children treated for ALL who did not undergo cranial irradiation include problems with memory, visuospatial/motor skills, processing speed, and attention. Less frequent deficits involve visuospatial nonverbal and verbal and auditory memory. Neurotoxic effects of L-asparaginase include an acute encephalopathy characterized by somnolence, lethargy, disorientation, seizures, and coma, which may be related to hepatic dysfunction and hyperammonemia in addition to the rare complications of coagulopathy.
Imaging
CT
In the settings of acute and subacute MTX neurotoxicity, CT is usually normal. In children with chronic MTX neurotoxicity, CT usually shows diffuse cerebral atrophy and low density within the cerebral hemispheric white matter (Fig. 44-1A). MTX-induced microangiopathic leukoencephalopathy is characterized by diffuse white matter low density with scattered calcifications within the deep white matter (see Fig. 44-1B). The white matter lesions typically do not enhance and have no mass effect, although rare cases of tumefactive ring-enhancing lesions simulating brain abscess have been reported. When high-dose MTX therapy is complicated by superimposed meningitis, the progression of white matter disease and calcification may be rapid and atypical in distribution (Fig. 44-2).
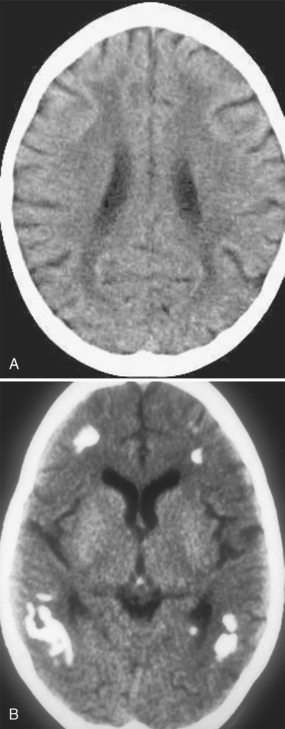
FIGURE 44-1 A, White matter changes from methotrexate toxicity. Nonenhanced CT shows diffuse low density within the deep and periventricular white matter; there is mild diffuse cerebral atrophy. B, Mineralizing angiopathy. Nonenhanced CT shows dystrophic calcification within the peripheral white matter.
(From Vazquez E, Lucaya J, Castellote A, et al: Neuroimaging in pediatric leukemia and lymphoma: differential diagnosis. RadioGraphics 2002; 22:1411-1428.)
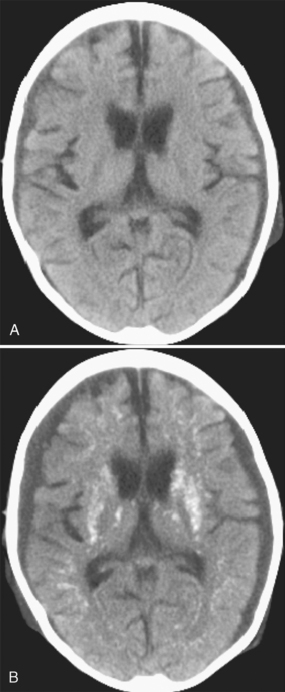
FIGURE 44-2 A, A 5-year-old girl with high-risk acute lymphoblastic leukemia and CNS involvement presented with Streptococcus sepsis 6 months after systemic and intrathecal chemotherapy; no cranial irradiation had been given. Nonenhanced CT at admission shows small, bilateral, moderately dense subdural collections, generalized cerebral atrophy, and no intraparenchymal calcification. B, Nonenhanced CT 8 days later shows the bilateral subdural empyemas and extensive parenchymal calcification.
MRI
The MR appearance of acute MTX neurotoxicity includes regions of restricted diffusion within the deep white matter of the cerebral hemispheres with little or no corresponding areas of abnormal T2 signal (Fig. 44-3). The areas of restricted diffusion do not correspond to a vascular distribution, do not enhance, and have no mass effect. The diffusion abnormalities may resolve completely or be followed by areas of abnormal increased T2 signal.3–8 Occasionally, there may be restricted diffusion within the cortex and/or leptomeningeal enhancement (see Fig. 44-3). Transient T2 prolongation in the cerebellar white matter and cerebral cortex has also been described. From 15% to 75% of patients imaged during treatment for ALL show white matter abnormalities; the incidence is highest at around 20 weeks after induction, during consolidation. The white matter T2-weighted (T2W)/FLAIR abnormalities may be bilateral and symmetric or asymmetric, usually spare the peripheral white matter, and are associated with some degree of generalized cerebral atrophy (Fig. 44-4). White matter lesions observed on conventional MRI are known to be temporary and reversible in some patients; however, progressive and persistent white matter changes may be seen in the absence of symptomatic neurotoxicity.5
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree