© Springer Science+Business Media New York 2016
Luca Saba and Eytan Raz (eds.)Neurovascular Imaging10.1007/978-1-4614-9029-6_493. Nonatherosclerotic Vascular Disease: Biological and Pathological Basis
(1)
Department of Radiological, Oncological and Anatomo-Pathological Sciences, Sapienza University of Rome, Viale Regina Elena 324, 00161 Rome, Italy
Abstract
Atherosclerosis is the main cause of vascular disease in most cases; in about 10 % of cases, carotid artery disease is related to nonatherosclerotic causes, including several unfrequent pathologies such as Takayasu arteritis, giant cell arteritis, fibromuscular disease, moyamoya syndrome, arterial dissection and extracranial carotid aneurysm. These entities are discussed in the present chapter, with a special focus on pathogenesis. Indeed, the aetiology of these diseases is in most cases not completely known, since related to several factors (genetic, immune and infectious). Early diagnosis, usually leading to a good patient’s outcome, is usually achieved after clinical examination and imaging tests.
Keywords
Arterial dissectionCarotid artery diseaseExtracranial carotid aneurysmFibromuscular dysplasiaGiant cell arteritisMoyamoya syndromeNonatherosclerotic diseaseTakayasu arteritisIntroduction
Atherosclerosis is identified as the main cause of vascular disease in around 90 % of cases; in the remaining 10 % of cases, nonatherosclerotic causes include several less common entities, such as Takayasu arteritis, giant cell arteritis, fibromuscular disease, moyamoya syndrome, arterial dissection, and extracranial carotid aneurysm. The pathogenesis of these diseases is in most cases unclear or even related to several factors, such as genetic, immune, and infectious, often associated with triggering events. The correct diagnosis is currently achieved after clinical examination and imaging tests; although US, CT, and MRA are useful, catheter angiography represents the gold standard in diagnosing most of these diseases. The majority of patients have a good outcome if the specific disease is diagnosed early.
Takayasu Arteritis (TA)
Takayasu arteritis (TA) is a granulomatous arteritis affecting the aorta and its branches [1]. The first case was reported by Takayasu in 1905 [2]; lately, the disease was more comprehensively described as “pulseless disease” by Shimizu and Sano in 1951 [3]. It generally affects women in the first fourth decades of life (nine females/one male), with a general incidence of 2.6 cases per million per year in the USA and major prevalence in patients of Asiatic origin [4].
The etiology of TA is still unclear; the underlying pathologic process is inflammatory, with several etiologic factors, either infective or autoimmune, having been proposed. The most likely hypothesis is that an unknown stimulus triggers the expression of the 65 kDa heat-shock protein in the aortic tissue which induces the major histocompatibility class I chain-related A (MICA) on vascular cells. The T cells and NK cells recognize MICA on vascular smooth muscle cells and release perforin, resulting in acute vascular inflammation; pro-inflammatory cytokines are also released and increase the recruitment of mononuclear cells within the vascular wall. Th1 lymphocytes drive the formation of giant cells through the production of interferon-γ and activate macrophages with release of VEGF resulting in increased neovascularization and PDGF, resulting in smooth muscle migration and intimal proliferation. The inflammatory cellular infiltrate mainly involves the media and adventitia, usually characterized by three stages: [5, 6].
1.
A systemic stage, characterized by signs and symptoms of an acute inflammatory condition, such as fever, arthralgia, anemia, and increased erythrocyte sedimentation rate
2.
A vascular inflammatory stage, when vascular stenosis and less frequently aneurysms occur, with corresponding signs and symptoms (stroke, transitory ischemic attacks, hypotensive ischemic retinopathy with visual symptoms, vertebrobasilar ischemia, hypertensive encephalopathy)
3.
A burned-out stage, when fibrosis sets, usually associated with remission
According to the American College of Rheumatology (ACR) [7], the criteria for assessing the diagnosis are:
Age at disease onset <40 years
Claudication of extremities
Decreased brachial artery pulse
BP difference >10 mmHg
Bruit over subclavian arteries or aorta
Arteriogram abnormality, represented by arteriographic narrowing or occlusion of the entire aorta, its primary branches, or large arteries in the proximal upper or lower extremities, not due to arteriosclerosis, fibromuscular dysplasia, or similar causes (changes usually focal or segmental)
A patient shall be said to have TA if at least three of these six criteria are present.
According to the classification proposed by Hata et al. [8], TA can be divided into six types based on angiographic involvement:
Type I – branches of the aortic arch
Type IIa – ascending aorta, aortic arch, and its branches
Type IIb – Type IIa region plus thoracic descending aorta
Type III – thoracic descending aorta, abdominal aorta, renal arteries, or a combination
Type IV – abdominal aorta, renal arteries, or both
Type V – entire aorta and its branches
Hence, only types I, IIa, IIb, and V usually involve the carotid arteries, whereas the other types more commonly involve the thoracic descending and abdominal aorta and the renal arteries.
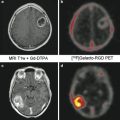
Angiography is the gold standard for diagnosis, even though color Doppler imaging can be useful in the first part of the diagnostic process, showing the mural thickening of the common carotid arteries (hypoechoic in the early stages and then hyperechoic after the development of fibrosis). CT, and especially MRA due to the young age of these patients, is also extremely helpful, even more during the follow-up [9]. If possible, whole-body MRI technique should be employed in order to exclude other localizations of disease [10].
TA is a chronic relapsing and remitting disorder. The overall 10-year survival rate is approximately 90 %, this rate being reduced in case of major complications, such as stroke, intracranial hemorrhage, and graft stenosis and/or occlusion. For this reason, strict management of traditional cardiovascular risk factors is mandatory in order to minimize secondary cardiovascular complications. Approximately 20 % of patients have a monophasic and self-limited disease; in the remaining 80 % of patients, TA requires immunosuppressive treatment, resulting in remission in around 60 % of cases [11].
Giant Cell Arteritis
Giant cell arteritis (GCA) , also known as temporal arteritis, is the most common form of vasculitis occurring in adults, with a prevalence of persons with active or remitted GCA of 200 cases per 100,000 population aged 50 years or older. The female-to-male ratio is 3.7:1 [12]. It is a granulomatous arteritis affecting large- or medium-sized vessels, usually the terminal branches of carotid arteries (more frequently the temporal and ophthalmic arteries). The clinical manifestations of giant cell arteritis result from two different processes. Inflammatory cells infiltrate the arterial wall and cause structural damage, eventually leading to vascular complications. In the majority of patients, a systemic inflammatory syndrome is also present. The systemic and the vascular components of giant cell arteritis seem to have different underlying pathogenetic mechanisms: the vascular inflammation results from abnormal adaptive immune responses, whereas the systemic inflammation likely depends on an excessively activated innate immune system [13]. Despite increased understanding of the inflammatory cascade responsible for the disease process, the initial event triggering the cascade remains uncertain; the adventitia is more likely considered as the site of initial immunologic injury [14].
According to the American College of Rheumatology [15], the criteria for assessing the diagnosis are:
Age at disease onset >=50 years
New headache
Temporal artery abnormality (temporal artery tenderness to palpation or decreased pulsation, unrelated to arteriosclerosis of cervical arteries)
Elevated erythrocyte sedimentation rate
Abnormal artery biopsy (biopsy specimen with artery showing vasculitis characterized by a predominance of mononuclear cell infiltration or granulomatous inflammation, usually with multinucleated giant cells)
A patient shall be said to have GCA if at least three of these five criteria are present.
The disease usually has an acute or subacute start, characterized by symptoms related to the acute inflammatory status (fever, night sweats) and headache, jaw pain, and blurred or double vision; if the disease is not early diagnosed, complications may occur, such as blindness and less frequently stroke [9].
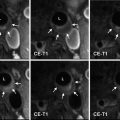
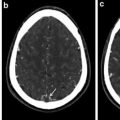
The diagnostic process starts with clinical examination and imaging studies. US findings can show swelling of the vessel’s wall as a hypoechoic dark halo around the color-coded flow in the artery; the disease is segmental; therefore, its visualization is suitable for localization of the biopsy [16]. CT and MR imaging can show an abnormal wall enhancement of the affected arteries after contrast media injection in the acute phase, whereas vascular stenosis and aneurysm can be observed in the subacute-chronic phase. In the chronic phase, the differential diagnosis between GCA and atherosclerotic disease may not be easy, but the different localization of the abnormalities (temporal and ophthalmic arteries for GCA, ubiquitarious for atherosclerosis) can lead to the correct diagnosis. Standard test for definitive diagnosis is biopsy of the temporal artery, being more samples needed because the inflammation usually does not involve all parts of the artery [10].
The prognosis for patients with untreated GCA is extremely poor; these patients may suffer blindness or death from myocardial infarction, stroke, or dissecting aortic aneurysm. On the contrary, with prompt and adequate therapy, full recovery is the rule. Symptoms from temporal arteritis usually improve within days of treatment with corticosteroids, except for those symptoms related to an effective vision damage that occurred before initiation of therapy, which are often irreversible. The mean duration of treatment is 2 years [17].
Fibromuscular Dysplasia (FMD)
Fibromuscular dysplasia (FMD) is an angiopathy affecting medium-sized arteries, more frequently in young women of childbearing age. Among patients with identified FMD, renal involvement occurs in 60–75 % and cerebrovascular involvement in 25–30 %; involvement of visceral arteries and arteries of the limbs is less commonly observed (about 9 % and 5 %, respectively); in the case of cerebrovascular localization, the internal carotid artery is more frequently affected (C2 segment) [9, 18].
The etiology of FMD is not known, even though several factors have been considered as involved in its pathogenesis. More in detail, hormonal factors such as estrogen have been proposed; however, although in the US Registry 91 % of registrants were female, clear supporting epidemiological evidence for the role of female hormones beyond the sex and age distribution of FMD has not been described yet. Although no etiologic genes for FMD have been identified, evidence supports a genetic basis for susceptibility to FMD; a few authors reported the potential inheritance pattern to be autosomal dominant with variable penetrance. However, several factors have limited the identification and characterization of genes contributing to FMD, such as disease rarity, variable phenotype, and gene-environment interactions [19]. FMD is usually described in terms of the affected arterial layer and the composition of the lesions; depending on the type of FMD, the narrowing (stenosis) of the artery is caused by an excess of either the fibrous or muscular components of the arterial wall [18].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree