Iatrogenic
Central venous access device (CVAD)
Cardioverter defibrillator/pacemaker
Ventriculoatrial shunt
Postoperative
Cervical manipulation
Neoplastic
Mass effect
Primary tumor
Lymphadenopathy
Direct invasion
Migratory thrombophlebitis (Trousseau syndrome)
Posttreatment (chemotherapy and/or radiation therapy)
Infectious
Intravenous drug abuse (IVDA)
Human necrobacillosis (Lemierre syndrome)
Otomastoiditis
Postauricular abscess
Cytomegalovirus
Traumatic
Blunt trauma
Penetrating trauma
Hematologic
Activated protein C (APC) resistance
Congenital
Factor V Leiden thrombophilia
Factor V Cambridge mutation
Acquired
Antiphospholipid antibodies (APLAs)
Oral contraceptives
Paraneoplastic
Thalidomide
Hemophilia therapies
Factor VIII inhibitor bypass activity (FEIBA)
Activated recombinant factor VII (rVIIa)
Rheumatologic
Rheumatoid arthritis
Systemic lupus erythematosus
Silk road disease (Behçet disease)
Gynecologic
Assisted reproductive techniques (ART)
In vitro fertilization
Ovarian hyperstimulation syndrome (OHSS)
Pregnancy
Endocrine
Tertiary or secondary hypercortisolism (Cushing syndrome)
Thyroid carcinoma
Goiter
Idiopathic
Pediatric patients
Iatrogenic
CVAD
Intravenous immunoglobulin (IVIg) infusion
Infectious
Human necrobacillosis (Lemierre syndrome)
Mastoiditis
Neoplastic
Hematologic
Traumatic
Congenital heart disease
Cystic fibrosis
Given the many etiologies of JVT, it is important for radiologists to consider the clinical context with this imaging finding, as well as the appearance on different imaging modalities. In doing so, one can suggest an appropriate, patient-specific, clinically relevant differential diagnosis and add real clinical value to a radiologic report. A comprehensive search for the etiology of JVT will often rely on a thorough review of the electronic medical record and clinical consultation, as well as scrutinizing other radiologic studies for imaging clues. The diagnosis of JVT, like so many other diagnoses encountered in diagnostic imaging, is an investigational starting point and not simply a dead-end observation.
Anatomy
The paired IJVs serve as the primary conduit for venous return not only from the superficial parts of the face and neck but also from the intracranial circulation to the right atrium of the heart (Fig. 1). Functionally, the IJVs are a continuation of the sigmoid sinuses below the level of the jugular foramina in the skull base. The left IJV is generally smaller than the right, and there is a unilateral valve in most cases [1].
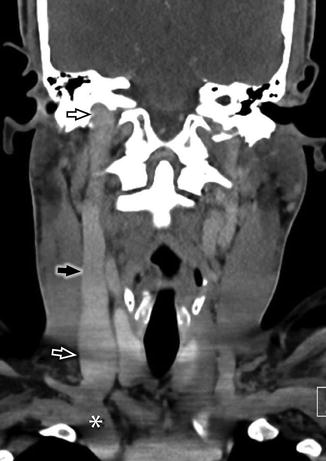

Fig. 1
Coronal reconstruction of CECT of the neck demonstrates a normal appearance of the right IJV (black arrow), which extends from the confluence of the subclavian and brachiocephalic veins (white asterisk) centrally to the sigmoid sinus peripherally. Normal, focal dilatations at the cranial and caudal extremes of the IJV are known as the superior jugular bulb (black open arrow) and inferior jugular bulb (white open arrow), respectively
At its cranial terminus is a normal, focal dilatation of the IJV, which is known as the superior bulb (Figs. 1 and 2). The superior jugular bulb typically lies below the floor of the internal auditory canal (IAC), but those extending above the floor of the IAC are considered “high-riding.” Although a high-riding jugular bulb is a clinically silent finding, it may be at increased risk of injury during mastoidectomy. In comparison, a dehiscent jugular bulb occurs when rarefaction of the osseous covering allows extension of the jugular bulb into the middle ear cavity. One may also encounter a jugular bulb diverticulum variant, which is a tubular projection of the jugular bulb into the adjacent skull base without extension into the middle ear.
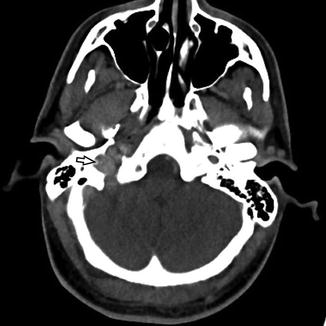

Fig. 2
Typical appearance of the superior jugular bulb (black open arrow), a normal focal dilatation of the IJV
Below the skull base, the IJV resides within the carotid space, deep into the sternocleidomastoid muscle. In the suprahyoid neck, the IJV is typically located posterolateral to the internal carotid artery (ICA) (Fig. 3), overlying the rectus capitis lateralis, and the glossopharyngeal and hypoglossal nerves pass between the ICA and IJV, while the vagus nerve lies posteriorly. In the infrahyoid neck, the IJV is typically located lateral and anterolateral to the internal carotid and common carotid arteries (Fig. 4). However, the exact location of the IJV with respect to the carotid artery is somewhat variable, particularly at the extremes of age [2]. At its caudal terminus, the IJV joins the subclavian vein and forms the brachiocephalic vein. Just proximal to its termination is a second dilatation, the inferior jugular bulb (Figs. 1 and 5). Numerous venous tributaries commonly feed into the IJV, including the facial vein, retromandibular vein, superior thyroid vein, and middle thyroid vein, although there is great variation and overlap of tributary venous return.
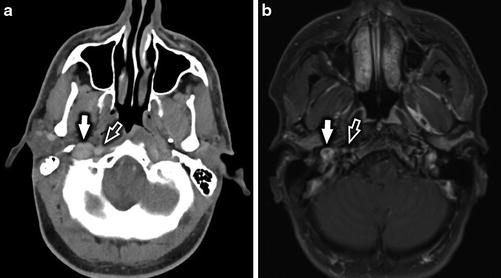
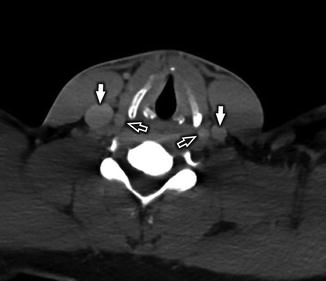
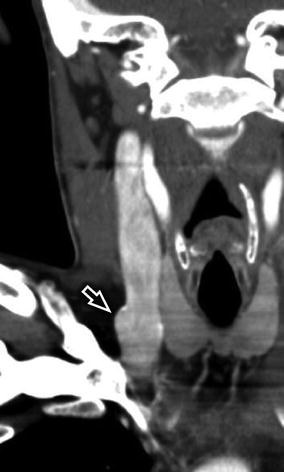

Fig. 3
Normal anatomy of the IJV in the suprahyoid neck. Axial CECT (a) and axial T1-WI +C (b) at the level of the nasopharynx demonstrate the IJV (white arrow) to be located lateral to the ICA (white open arrow)

Fig. 4
Normal anatomy of the IJV in the infrahyoid neck. Axial CECT at the level of the glottic larynx demonstrates the IJVs (white arrows) to be located lateral to the internal carotid arteries (white open arrows). While typically located lateral or anterolateral to the ICA in the infrahyoid neck, the location of the IJV can be variable

Fig. 5
Coronal CECT image demonstrates the inferior jugular bulb (white open arrow), a normal, focal dilatation of the IJV just before its confluence with the jugular vein
Pathophysiology
The pathophysiology of JVT is rooted in the well-known Virchow triad. This is named after, but probably not actually described (at least in its current understanding), by Rudolf Ludwig Karl Virchow (1821–1902), the so-called father of modern pathology; this model posits that three broad categories of factors are at play in the development of vascular thrombosis: static blood flow, endothelial injury, and hypercoagulability [3] (Fig. 6). An abnormality of one or more of these variables increases the risk of developing a thrombus. In its native state, the IJV, like all other veins of the head and neck, is an unlikely culprit to develop thrombus, due in large part to its paucity of valves and the aid of gravity in venous return while the body is in an upright state [4]. While in the supine position, the IJVs serve as the primary venous drainage pathway for the brain; in the erect position, these veins collapse and flow via alternate vertebral venous pathways [5]. For these reasons, spontaneous JVT is rare and must raise clinical concern for an underlying culprit causing static blood flow, endothelial injury, or hypercoagulability.
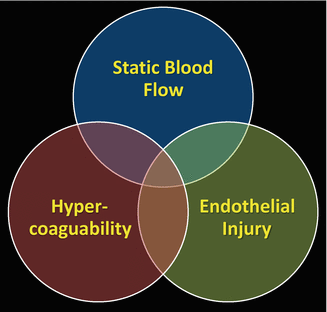

Fig. 6
An abnormality in one or more of these variables increases the risk of developing a thrombus. Areas of intersection of the circles in this Venn diagram reflect increased risk of thrombosis. At highest risk are patients who fall into all three categories
However, certain patient populations are at increased risk of altering the dynamics of the Virchow triad, shifting them toward a prothrombotic state and subsequent JVT (Table 2). For example, patients with central venous access devices (CVADs) are at increased risk of JVT due to static venous flow along the catheter as well as due to endothelial injury from catheter placement. Patients with a history of intravenous drug abuse (IVDA) are at increased risk of JVT from either caustic, chemical endothelial injury, or endothelial injury from IVDA-associated blood-borne infection. Patients with underlying neoplasm may be at increased risk for JVT formation due to a systemic, cancer-induced hypercoagulable state or from compression of the vessel lumen by cervical lymphadenopathy or primary tumor, resulting in static flow and impaired venous return. These few examples illustrate how the components of the Virchow triad contribute to thrombosis of this ordinarily resilient vessel. Keeping these basic principles in mind during image interpretation can be quite helpful in deciphering the etiology of JVT in specific clinical settings.
Table 2
High-risk patient populations for development of JVT
Central venous access device (CVAD) |
Intravenous drug abuse (IVDA) |
Malignancy |
Clinical Presentation
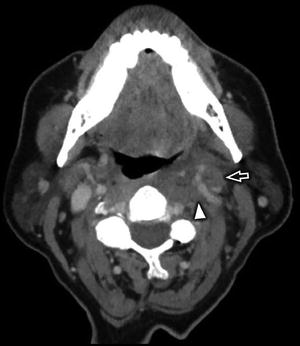
Just as the etiologies of JVT are diverse and numerous, so too can be the clinical presentation (Table 3). Presentation may range from completely asymptomatic (incidental) to palpable neck lump, dysphagia, cervical edema, upper extremity edema, erythrocyanosis, varicose superficial collateral veins, pulmonary embolism, or frank sepsis, depending on the etiology, acuity, and extent of disease [6, 7]. Patients more commonly present with unilateral disease, although bilateral disease is not infrequently encountered (Fig. 7). During the acute and subacute phases (typically within 10–14 days of presentation) of jugular vein thrombophlebitis, patients may experience diffuse edema and tenderness overlying the sternocleidomastoid muscle. Patients are often suspected of having an abscess. In the chronic phase after the inflammation subsides, thrombus may persist within the internal jugular vein, resulting in a palpable, cord-like mass and in many cases total occlusion.
Table 3
Clinical presentation of jugular vein thrombosis
Acute phase (first 2 weeks) |
Asymptomatic |
Palpable lump |
Neck pain |
Dysphagia |
Cervical or upper extremity edema |
Erythrocyanosis |
Varicose collateral veins |
Pulmonary embolism |
Sepsis |
Chronic phase (>2 weeks) |
Palpable, cord-like mass |
Varicose collateral veins |

Fig. 7
Sixty-year-old man with left ventricular access device (LVAD) who presents with altered mental status concerning for acute cerebral infarct. Coronal reformat of CTA of the neck demonstrates occlusive thrombus in the left IJV (white arrow) and near-complete occlusive thrombus in the right IJV (white open arrow)
Etiologies
In order to systematically cover the many possible etiologies for JVT, it is helpful to first consider the diagnosis from a “bird’s-eye view,” compartmentalizing the potential sources into several general categories – iatrogenic, neoplastic, infectious, traumatic, hematologic, rheumatologic, gynecologic, endocrine, and idiopathic – which will be discussed individually. Potential etiologies of JVT in the pediatric population will also be discussed.
Iatrogenic
Given the widespread use of CVADs, the prevalence of head and neck surgery, and the increased use of transvenous medical devices, iatrogenic sources are the most common causes of JVT in modern medicine [8–10]. IJV occlusion (typically non-thrombotic) is infrequently seen postoperatively following head and neck surgery (Figs. 8–15). Among iatrogenic causes, the vast majority of JVT is due to central venous catheters, often resulting in “uphill thrombosis” that propagates into the IJV from the downstream subclavian or brachiocephalic veins (Fig. 16). At-risk patient populations include those with chronic indwelling central venous catheters, as might be utilized for chemotherapy or frequent blood draws, and critically ill neonates requiring central venous access. JVT as a complication of other transvenous biomedical devices, such as cardioverter defibrillators, pacemakers, and ventriculoatrial shunts, is seen less commonly but has been reported in the medical literature [9, 10]. As previously described, most iatrogenic causes of JVT center around catheter-/device-induced venous stasis and endothelial injury, although it is important to consider superimposed hypercoagulability in these typically ill patients.
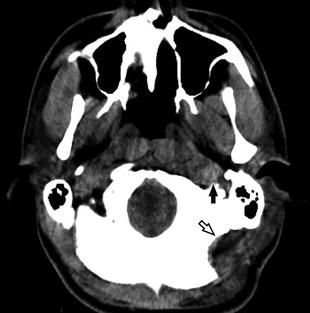
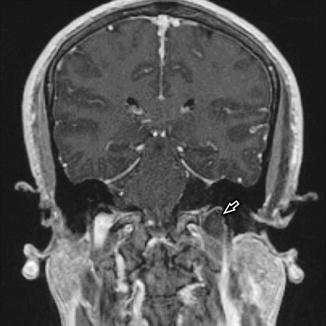
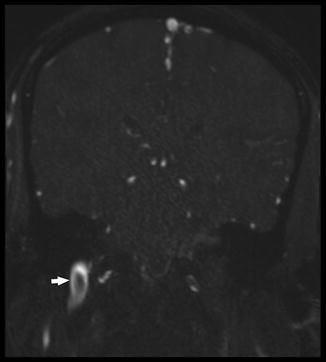
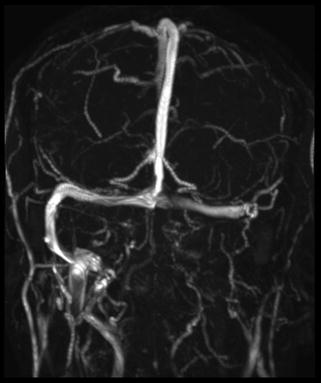
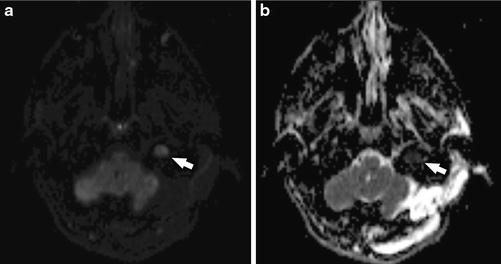
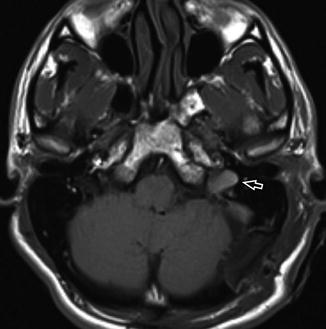
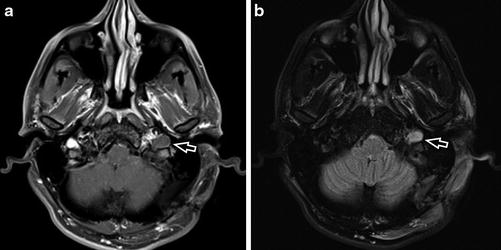
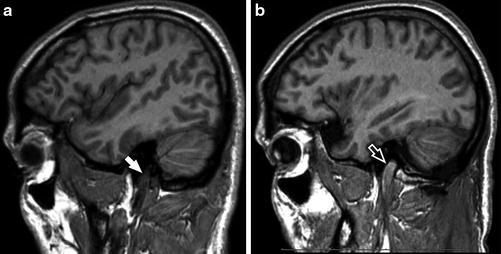
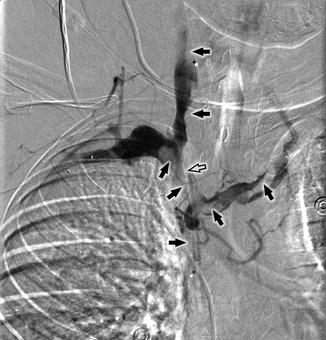

Fig. 8
Postoperative NECT in a 31-year-old man with recent left retrosigmoid craniectomy (black open arrow) for resection of a vestibular schwannoma demonstrates high density within the left IJV (black arrow), corresponding to “downhill thrombus” propagating from the thrombosed sigmoid sinus

Fig. 9
Volumetric T1-WI +C in the same patient as Fig. 8 demonstrates a low signal intensity filling defect (white open arrow) within the enlarged left jugular bulb, corresponding with acute postoperative “downhill” JVT that had propagated from the sigmoid sinus

Fig. 10
Coronal 2D TOF image in the same patient as Figs. 8 and 9 demonstrates apparent filling defect (white arrow) in the right IJV which is due to turbulent blood flow and should not be confused with true thrombus. In equivocal cases, further evaluation with dynamic post-contrast MR venography or second-look ultrasound can be considered for clarification

Fig. 11
Coronal maximal intensity projection (MIP) from 2D time-of-flight (TOF) MRV in the same patient as Figs. 8, 9, and 10 shows no flow-related enhancement within the distal left transverse sinus, sigmoid sinus, jugular bulb, and visualized IJV, corresponding with “downhill” thrombus following left retrosigmoid craniectomy


Fig. 13
Axial T1-WI −C demonstrates the left JVT (white open arrow) to be isointense to brain parenchyma and slightly hyperintense to skeletal muscle

Fig. 14
Axial T1-WI +C (a) and axial T2-WI FS (b) in a 31-year-old man with recent left retrosigmoid craniectomy for resection of a vestibular schwannoma demonstrates “downhill” JVT (white open arrow) that has propagated from iatrogenic thrombosis of the left sigmoid sinus. Note that the thrombus is isointense to slightly hyperintense to brain parenchyma on T1-WI +C and T2-WI FS and demonstrates a thin rim of peripheral enhancement on post-contrast imaging due to enhancement of engorged vasa vasorum

Fig. 15
Sagittal SPGR-C imaging through the bilateral IJVs in the same patient demonstrates the normal, low signal (white arrow) within the patent right IJV (a) and increased signal (white open arrow) within the thrombosed left IJV (b)

Fig. 16
Digital subtraction angiography (DSA) of the right upper extremity in a 45-year-old woman with “uphill thrombosis” of the right IJV demonstrates filling defects (black arrows) within the right IJV, subclavian vein, right brachiocephalic vein, left brachiocephalic vein, and superior vena cava. A potentially thrombogenic central venous catheter is present (black open arrow)
A large group of patients with increased susceptibility to iatrogenic JVT is hemodialysis (HD) patients, as they tend to have numerous, long-term CVADs placed over a lifetime (Fig. 17). Studies have demonstrated that in HD patients, JVT risk increases with both duration of catheter placement and total lifetime number of catheters placed, with subsequent JVT reported in 2–64 % of cases based on older studies [8, 11]. The underlying renal insufficiency itself is associated with hemostatic abnormalities, and HD adds to these disturbances with turbulent flow, high shear stress, and contact of blood to artificial surfaces. This leads to activation of the coagulation cascade, which is the target of anticoagulation agents, such as unfractionated heparin and low-molecular-weight heparin.
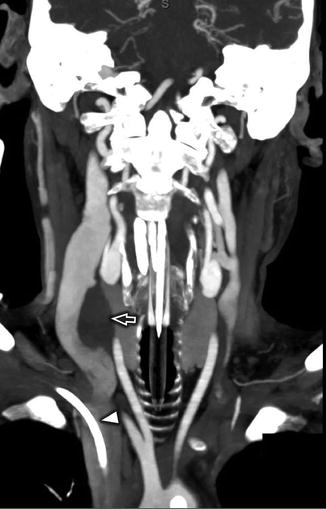

Fig. 17
Coronal MIP from CTA of the neck in a 60-year-old critically ill patient requiring hemodialysis delineates the nonocclusive thrombus in the right IJV (white open arrow), as well as a potentially thrombogenic right internal jugular venous catheter (white arrowhead)
Another subset of patients at risk for IJV thrombosis or occlusion is that group who has undergone neck surgery, most commonly following neck dissection for head and neck cancer. While IJV patency is dependent on the type and extent of neck dissection (with intentional sacrifice of the IJV performed in some cases), true thrombosis or complete occlusion following IJV-sparing neck dissection is uncommon [12]. However, a diminution in IJV caliber is expected following neck dissection, reaching a nadir in the immediate postoperative period and frequently returning to near-normal caliber by 3 months [12]. Uncommonly, JVT can be seen following carotid endarterectomy, presumably due to prolonged intraoperative retraction of the IJV.
Lastly, there are reports of JVT following cervical manipulation, including chiropractic and deep tissue massage. Other than case reports, the literature evidence for or against cervical manipulation-induced IJV thrombosis is certainly lacking. The mechanism of thrombus formation in these rare cases is not well elucidated, although it might theoretically reflect a combination of pressure-induced venous stasis and traumatic endothelial injury.
Neoplastic
Like lower extremity deep vein thrombosis (DVT), JVT is commonly associated with malignancy, with coincidence reported up to 10–35 % in some series [7, 13] (Figs. 18–21). Mechanistically, neoplasm-related thrombosis of the IJV results from (a) compression/invasion of the vessel lumen by lymphadenopathy or primary tumor, resulting in static blood flow, (b) a complex, incompletely understood cancer-induced hypercoagulable state (often referred to as migratory thrombophlebitis or Trousseau syndrome) that results in increased levels or activity of several procoagulant factors (including tissue factor, fibrinogen, factor VIIa, factor VIII, factor IXa, factor Xa, C-reactive protein, carcinoma mucins, tumor-associated cysteine proteinase, or oncogene activation), or (c) iatrogenic consequences of cancer treatment (cf. Iatrogenic) [4, 14, 15].
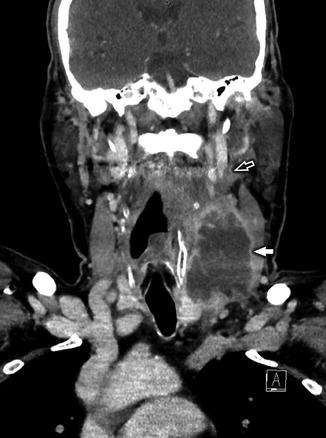
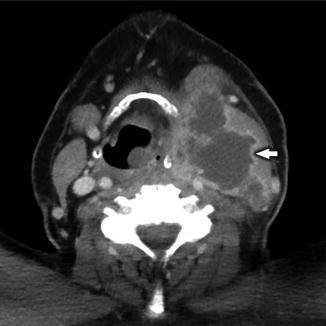
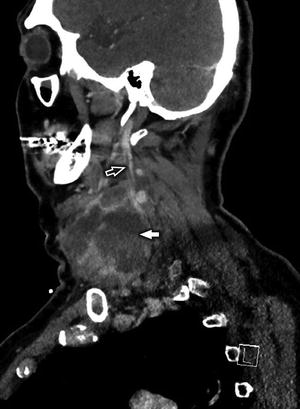

Fig. 18
Coronal reconstruction from a CECT of the cervical soft tissues in a 47-year-old man with laryngeal squamous cell carcinoma (SCCa) and necrotic, conglomerate nodal metastases (white arrow) demonstrates marked mass effect upon the left IJV and direct invasion with tumor thrombus (white open arrow)

Fig. 19
Axial CECT in the same patient as Fig. 18 demonstrates the necrotic conglomerate nodal metastases (white arrow) with extranodal spread of tumor, which completely obliterates the left IJV

Among all cancer patients, treatment-related causes are the most common etiology of JVT; these include CVADs, prolonged immobility, intravascular administration of caustic chemotherapeutic agents, postsurgical vascular injury, and postradiation therapy vascular complications. Nonetheless, the presence of JVT, especially in cancer patients, should prompt the interpreting radiologist to carefully assess the cervical lymph node stations for metastatic lymphadenopathy and to assess the deep spaces of the neck for a lesion exerting mass effect on the thrombosed IJV. While most often due to underlying head and neck cancer (such as squamous cell carcinoma of the aerodigestive tract or thyroid malignancy), cervical chain lymphadenopathy causing JVT can also be seen in widely disseminated metastatic disease of any number of primary malignancies [16–18]. The most common of these primary malignancies include pancreatic, lung, stomach, and ovarian cancer, which may contribute to JVT due to increased factor VIII and thromboplastin levels. Additionally, nonmalignant neoplasms, such as lymphangioleiomyomatosis, can lead to JVT due to local mass effect and resulting venous stasis. Both contrast-enhanced CT (CECT) and ultrasound (US) are useful modalities for assessment of the neck masses, with US offering the opportunity for image-guided fine needle aspiration or core needle biopsy of suspicious masses or lymphadenopathy.
Infectious
Prior to the widespread use of antibiotics in the 1940s, infection was by far the most common etiology of JVT. However, in current medical practice infectious sources of JVT are quite rare, attributed mainly to IVDA (Figs. 22–24) and sporadic cases of typically oropharyngeal infections resulting in Lemierre syndrome. IVDA increases the risk of JVT for several reasons: (1) shooters typically do not employ sterile technique, thus risking blood-borne infection; (2) traumatic endothelial injury from the needle encourages thrombosis; (3) infectious (mycotic) endothelial damage by blood-borne organisms results in prothrombotic infiltration and congestion of the adventitia (and subsequently the other layers of the vein wall) by inflammatory cells; and (4) IVDA-associated deep neck infection contributes to altered blood flow dynamics, including venous stasis [19]. Other infectious causes of the deep spaces of the head and neck, such as postsurgical infection (Figs. 25–33), acute otomastoiditis, or postauricular abscess, may also contribute to the development of JVT, albeit much less commonly. Additionally, an increased risk of JVT in immunocompetent patients with severe acute cytomegalovirus infection has been described, a link which may be under-recognized and underreported.
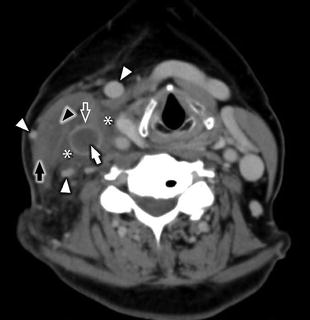
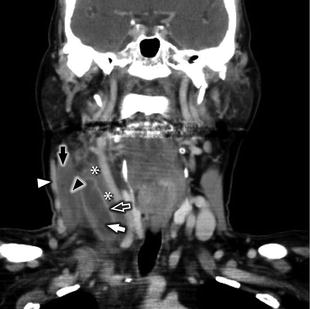
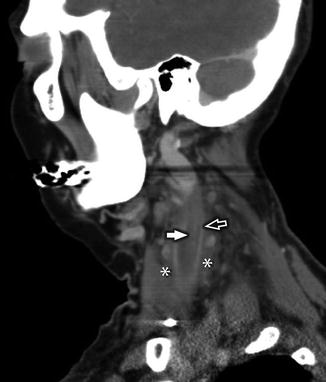
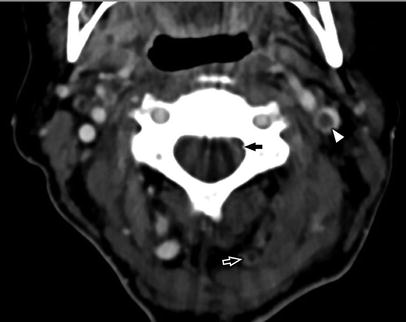
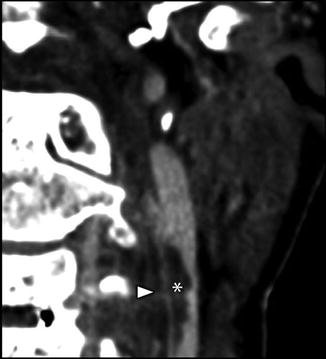
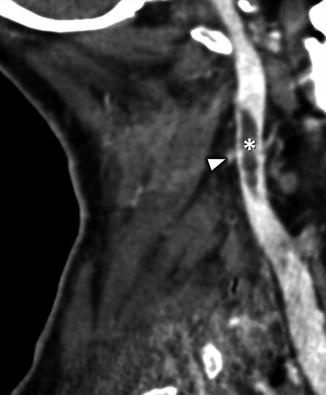
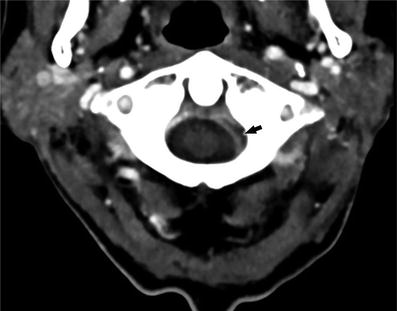
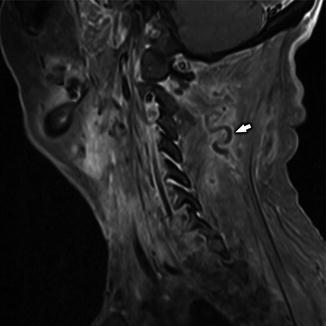
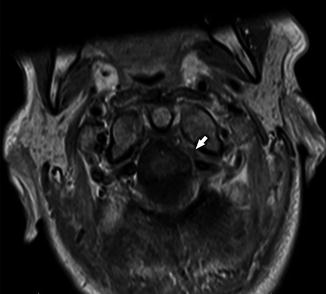
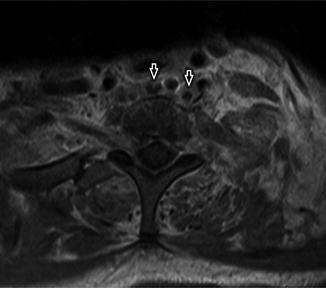
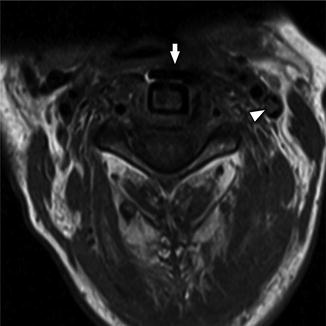
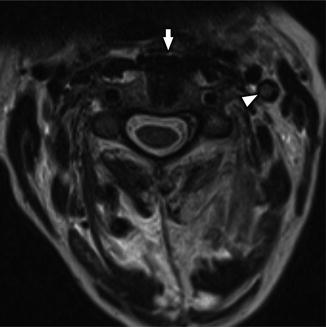

Fig. 22
Axial CECT in a 50-year-old woman with acute catheter-associated JVT and septic thrombophlebitis, who presented with 6 days of right-sided neck pain, swelling, and fever. Image demonstrates complete occlusion of an enlarged right IJV with central low-attenuation filling defect (white arrow), prominent enhancement of the vasa vasorum (white open arrow), perivascular edema (white asterisks), subtle intramuscular enhancement (black arrowhead) within an enlarged, edematous right sternocleidomastoid muscle (black arrow), and multiple venous bypass collaterals (white arrowheads)

Fig. 23
Coronal reformat of CECT in the same patient as Fig. 22 demonstrates complete occlusion of an enlarged right IJV with central low-attenuation filling defect (white arrow), prominent enhancement of the vasa vasorum (white open arrow), perivascular edema (white asterisks), intramuscular phlegmon/abscess (black arrowhead) within an enlarged, edematous right sternocleidomastoid muscle (black arrow), and prominent venous bypass collateral (white arrowhead)


Fig. 25
Axial CECT in a 76-year-old woman with recent anterior cervical fusion presents with neck pain and fever. Image reveals central filling defect in the left IJV (white arrowhead) compatible with JVT. Additional thrombus was seen in the left ventral epidural venous plexus (black arrow) and left-sided muscular collateral veins (white open arrow)

Fig. 26
Coronal reconstruction of the NECT data in the same patient as Fig. 25 better delineates the irregular, low-attenuation filling defect (white asterisk) seen eccentrically within the left IJV as well as peripheral wall enhancement corresponding to engorged vasa vasorum (white arrowhead)

Fig. 27

Fig. 28
Axial CECT at the C1 level demonstrates a filling defect (black arrow) within the left ventral epidural space corresponds with additional thrombus

Fig. 29
Sagittal T1-WI +C demonstrates filling defect within a left-sided muscular collateral vein (white arrow) as well as peripheral enhancement within the vessel resulting from enhancement of the vasa vasorum

Fig. 30
Axial T1-WI +C demonstrates filling defect (white arrow) within the ventral left epidural space at C1–C2, compatible with thrombus in the epidural venous plexus

Fig. 31
Axial T1-WI +C in the same patient as Fig. 30 demonstrates peripherally enhancing fluid collections (black open arrows) in the prevertebral soft tissues, compatible with small abscesses

Fig. 32
Axial T1-WI +C demonstrates a filling defect within the left IJV (white arrowhead), which is isointense to skeletal muscle. The patient has recently undergone anterior cervical fixation (white arrow)

Fig. 33
Axial T2-WI in the same patient as Fig. 32 illustrates a filling defect within the left IJV (white arrowhead) which is slightly hyperintense to skeletal muscle. The patient has recently undergone anterior cervical fixation (white arrow)
Lemierre Syndrome
A unique subset of JVT that warrants special consideration is Lemierre syndrome (LS), also known as human necrobacillosis or anaerobic postanginal sepsis. Although not originally described by French bacteriologist André Lemierre (1875–1956), this eponymous distinction was awarded to him as a result of his detailed description of systemic manifestations of Bacillus funduliformis (now called Fusobacterium necrophorum) in 1936. Classically, LS is characterized by a constellation of clinical findings that include pharyngitis within the preceding 4 weeks, septic embolic disease (typically to the lungs), internal jugular vein thrombophlebitis, and isolation of bacteria (typically F. necrophorum) from the blood or another sterile body site.
Although most commonly associated with F. necrophorum (57 %) or other Fusobacterium species (33 %), LS has also been reported with Staphylococcus aureus (including methicillin-resistant), Streptococcus, Peptostreptococcus, Proteus, Bacteroides, Klebsiella, and Porphyromonas species [20]. While LS is most often associated with a head and neck site of infection (~75 %), including the tonsils (37 %), pharynx/upper respiratory tract (30 %), middle ear/mastoid (2 %), larynx (2 %), dental (1 %), paranasal sinuses (1 %), and orbit (1 %), approximately 25 % of cases originate in the chest or lower respiratory tract [20]. Extraordinarily rare cases of LS originating from the gastrointestinal tract, genitourinary tract, and musculoskeletal system have also been reported. Presenting symptoms typically relate to site of involvement, most commonly sore throat, neck pain, or neck mass. Several series have described LS to primarily affect previously healthy children, adolescents, and young adults, with an equal to slightly increased male-to-female predominance [20, 21].
LS is characterized by four distinct pathophysiologic phases: primary infection, invasion of the parapharyngeal (lateral pharyngeal) space, internal jugular vein thrombophlebitis, and metastatic (septic embolic) disease [22]. While disease progression often advances in a predictable, stepwise manner through these phases, it is not unexpected to find cases where metastatic complications, such as pulmonary disease, present before JVT or JVT appears before parapharyngeal disease. Early intervention with prolonged (multi-week) course of antibiotics is the current therapeutic standard [21, 22]. Therapeutic anticoagulation remains controversial, often reserved for patients with underlying prothrombotic disorder, secondary cerebral infarction, or associated cavernous sinus thrombosis [21]. Surgical intervention is typically limited to abscess drainage with IJV ligation rarely performed in current clinical practice.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree