Fig. 1
Age distribution of cases with childhood arterial ischemic stroke (n = 96) (From Mallick et al. [1] with permission)
Studies that included neonates have reported higher incidence rates of 2–3 per 100,000 per year. Neonates accounted for 8–46 % of total pediatric AIS cases across different studies, and perinatal stroke is as common as 1 in 2,500 live births [6]. It is not completely understood why the incidence rates of childhood arterial ischemic stroke vary with age, but different susceptibilities related to different underlying risk factors with age are likely relevant. Ethnicity background plays an important role in stroke risk with African and Asian children having a significantly higher incidence of stroke than Caucasian children [1]. This is partially due to sickle cell disease in African and moyamoya disease in Asian children.
Clinical Features and Diagnostic Delays
Any acute onset of a focal neurological deficit in a child is a medical emergency, with ischemic or hemorrhagic stroke being the most urgent diagnosis to exclude. Although the majority of children with CAIS will not be eligible for tPA, an early diagnosis is still essential to guide immediate neuroprotective and urgent antithrombotic treatments. However, despite an increasing number of international publications about CAIS and increased awareness of the entity over the last decade, the diagnosis of stroke in children is still frequently delayed or missed. A diagnostic delay of 24 h and more from symptom onset is not uncommon even when the child is brought to medical attention within the first 6 h after symptom onset [7]. Diagnostic delay is frequently maximal in the period following hospital arrival. Besides a lack of awareness of CAIS among parents and health professionals, a complex differential diagnosis with a wide spectrum of common stroke mimics occurring in up to 30 % of apparent strokes contributes to the diagnostic confusion. In addition, in children with definite CAIS, a falsely negative CT scan, still often the first imaging study performed, has been reported in up to 84 % [7]. This has resulted in a move to urgent MRI as initial imaging for CAIS.
Acute hemiparesis is the most common presenting focal deficit. However, a variety of other focal neurological deficits have been described in CAIS (Table 1). Unlike in adulthood, seizures at stroke onset are frequent in children [1]. Age-related differences in clinical presentation have to be considered. Especially in younger children, infants, and neonates where over three-quarters present with only seizures, symptomatology can be very nonspecific and misleading. CAIS may occur in the context of an acute illness, such as meningitis or post cardiac surgery, when clinical evaluation can be particularly challenging.
Table 1
Presenting signs and symptoms of arterial ischemic stroke in different age groups (Adapted from Mallick et al. [1])
<1 year (%) | 1–5 years (%) | 6–10 years (%) | 11–15 years (%) | |
|---|---|---|---|---|
Focal features | 75 | 89 | 70 | 91 |
Hemiparesis | 69 | 85 | 60 | 52 |
Facial weakness | 25 | 47 | 40 | 39 |
Speech disturbances | 13 | 32 | 40 | 48 |
Diffuse features | 63 | 47 | 100 | 74 |
Decreased conscious level | 60 | 36 | 50 | 39 |
Headache | 0 | 13 | 50 | 52 |
Seizures | 75 | 26 | 20 | 9 |
Imaging
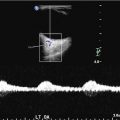
Urgent neuroimaging is indicated to establish the diagnosis in every case of a child presenting with an acute neurological deficit that is not otherwise sufficiently explained. Neuroimaging will also help to rule out complications, such as cerebral edema or intracranial hemorrhages, and possibly establish the etiology. Importantly, in young infants with an open fontanel, cranial ultrasound (CUS) is not reliable and, due to wide operator variability and a peripheral location of many infarcts, frequently missed the diagnosis of CAIS [8].
Computed Tomography
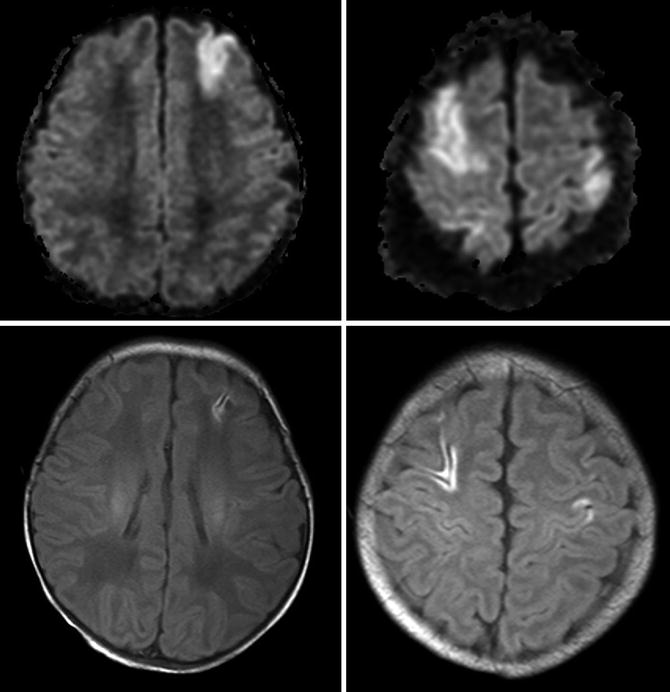
MRI is superior to CT in detecting strokes in children (Table 2). This is particularly true for early and small strokes and those located in the posterior fossa. However, immediate access to an MRI is not always feasible and additional sedation or anesthesia may be required compared with CT leading to delayed imaging. Therefore, CT still has a place in the acute diagnostic process. A head CT scan can rule out intracranial hemorrhage (ICH) and may identify subtle findings suggestive of stroke. As in adults the main CT findings are areas of cortical–subcortical hypoattenuating within a vascular territory, obscuration and loss of gray matter–white matter differentiation in the basal ganglia, and demonstration of a “hyperdense artery sign” (Fig. 2). CT may also reveal hemorrhagic transformation or malignant infarct edema. However, sensitivity to early ischemia is generally low [7] (Fig. 3). The presence of a persistent arterial occlusion on CTA can also suggest the diagnosis.
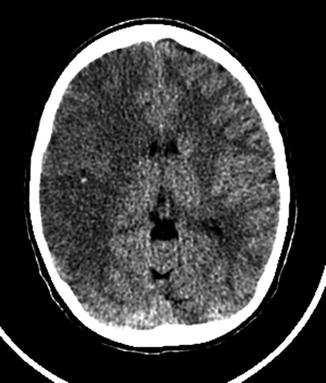
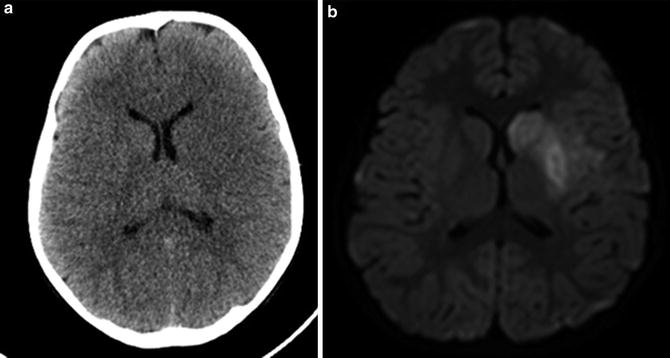
Table 2
Advantages of MR imaging versus computed tomography in childhood
MR imaging | Computed tomography |
|---|---|
Less widely available | Widespread access |
Extended acquisition time; sedation may be needed | Very fast acquisition speed |
No radiation | Uses ionizing radiation |
Diffusion-weighted imaging highly sensitive and specific for early stroke | Misses stroke diagnosis in the majority of children |
Gradient-echo imaging sensitive for detection of hemorrhage | Highly sensitive for detection of acute hemorrhage |
MR angiography (TOF) does not require contrast | CT angiography requires intravenous contrast |
TOF angiography is prone to flow artifacts | CT angiography provides more accurate anatomic illustration of cerebral arterial system |
Susceptibility-weighted images provide valuable information about cerebral venous system | Imaging of cerebral venous system requires contrast |
Mismatch studies (core vs. penumbra) usually not available in pediatric centers | Mismatch studies (core vs. penumbra) usually not available in pediatric centers |

Fig. 2
Hyperdense artery sign

Fig. 3
CT versus MRI of the same patient, 4 and 6 h after presentation with acute hemiparesis, respectively. The CT had been reported as normal
Modern CT techniques such as perfusion CT are not readily available in pediatric radiology departments. Furthermore, its use in childhood is generally discouraged because of an excessive radiation dosage. Therefore, compared to adults, the role of an acute head CT to diagnose CAIS in the pediatric population is limited.
Magnetic Resonance Imaging
A high-priority MRI is the preferred imaging technique for diagnosing acute stroke. Besides a high sensitivity and specificity to identify early strokes, identification of associated treatable conditions and exclusion of stroke mimics (see below) pose other important advantages. By comparing different MRI sequences, e.g., diffusion weighted (DWI) and FLAIR, the age of ischemic lesions from within 7 days to older can be estimated. Other features including mass effect or atrophy can also aid in estimating lesion age. In older teenagers, if tPA is a therapeutic option priority, 1 MRI is indicated (within 1 h) to confirm CAIS versus other mimics. Otherwise an early MRI as early as possible but within 4 h after presentation is still recommended in order to initiate urgent antithrombotic and neuroprotective therapy in those with definite CAIS. This is feasible with most children. A specific hospital stroke protocol focusing on the most relevant findings of interest is useful (Definite ischemia? Recent vs. old lesion, bleeding? Arterial anatomy). A stroke protocol MRI can usually be completed within 10 min (Fig. 4).
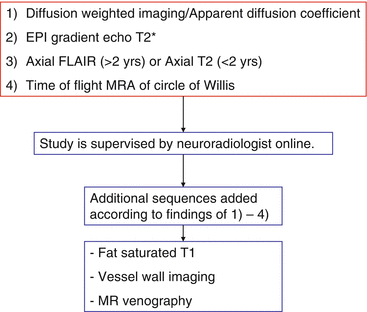

Fig. 4
Simplified stroke protocol. This approach allows exclusion of a stroke after a maximum of 10 min on the MR table
DWI is the gold standard for diagnosis of acute CAIS and has been proven helpful identifying ischemic lesions within hours in neonates and within minutes in older children [9]. Acute ischemic lesions demonstrate restricted diffusion, evidenced by hyperintensity on trace diffusion images. A matching reduced signal on apparent diffusion coefficient (ADC) map helps to discriminate recent from old lesions with so-called T2 shine-through. T2-WI and fluid-attenuated inverted recovery (FLAIR) images may show acutely infarcted tissue within 12–24 h of injury, and abnormalities will usually persist.
MRA of the intracranial and neck vessels help to identify and characterize an underlying arteriopathy. Vascular imaging is an essential part of cerebral imaging in all children with stroke (Fig. 5). Vascular imaging demonstrates an intra- or extracranial cerebral arteriopathy in about fifty percent of children presenting with an AIS [10].
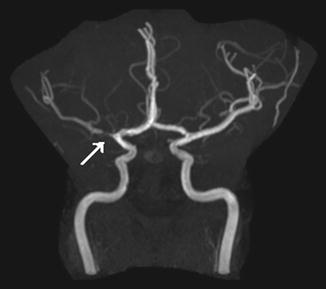

Fig. 5
Twelve-year-old girl with recurrent episodes of slurred speech and right arm weakness. MR angiography shows proximal M1 stenosis
However, technical limitations require consideration. Because MRA relies on flow signal, areas of normal (areas of branching or tortuosity) or abnormal (areas of stenosis) turbulent flow can mimic occlusions especially in infancy when arteries are smaller. TOF-MRA has been reported to overestimate the severity and length of a stenosis. In addition, MRA has its limitations in resolution, interfering with its ability to identify medium- or small-sized vessel diseases [11]. Gadolinium-enhanced MRA is superior to time of flight but not routinely performed in most pediatric centers.
SWI has recently been proven to be a useful MR sequence for CAIS for detecting hemorrhagic components within the region of infarction and identifying and quantifying microbleeds. In adults these have been shown to predict the risk of hemorrhagic transformation before initiating thrombolytic treatment. Preliminary data suggest that SWI may be helpful for visualization of the penumbra in children [12]. Potential pitfalls in SWI interpretation have been nicely summarized recently [13].
Conventional Angiography
Despite advances in noninvasive imaging techniques, conventional angiography (CA) remains the gold standard for detailed cerebral vascular imaging. Cerebral CA is the most sensitive and specific method of diagnosing most cerebral arteriopathies, many of which may only be identifiable with CA. CA also provides a dynamic view of the arterial perfusion including areas of low flow or, for collateral vessels and widened zones of perfusion into the ischemic territory, possible artery-to-artery embolism sources and distal narrowing or occlusion. CA may identify characteristic features of inflammatory and non-inflammatory vasculopathies such as dissection and is recommended when central nervous system vasculitis is suspected, especially when MR imaging is not conclusive. In addition, CA including both intracranial and extracranial carotid arteries is crucial for presurgical planning of revascularization, e.g., in cases of moyamoya disease.
Therefore, CA should be considered in all children with AIS in whom an etiology for CAIS remains elusive after initial MRA or CTA imaging. With pediatric expertise provided, the complication rate for diagnostic CA is low even in children below 3 years of age [14]. However, radiation remains a concern, and carefully weighing up the expected additional information and the risk of unnecessary exposure to radiation is warranted.
Patterns of Childhood Arterial Ischemic Stroke
Typical radiological features of AIS seen in children are similar to those of young adults. However, maturational changes that occur during brain development need to be taken into account. Imaging patterns of CAIS will depend on the location and the size of the vessels involved. Most CAIS involve the territory of the middle cerebral artery (MCA), most frequently affecting the left hemisphere [15]. The classical imaging appearance of occlusion of a large cerebral artery is the peripheral wedge-shaped infarct involving the cerebral cortex and subjacent white matter in territorial distributions.
The stroke pattern may differ according to the underlying etiology. As an example, large, isolated lenticulostriate AIS is commonly associated with parainfectious or inflammatory intracranial, unilateral arteriopathy including transient cerebral arteriopathy or post-varicella arteriopathy [16, 17]. Small lacunar infarcts are rare in childhood age groups except in cardiac or sickle cell disease.
Posterior Circulation Strokes
The vertebrovascular or posterior circulation is involved in up to 37 % of all CAIS cases [18], and boys are more commonly affected. Typical posterior circulation stroke patterns include occlusions of basilar artery and its branches (Fig. 6), proximal posterior cerebral artery (PCA) occlusion with large-vessel territory infarction in the occipital and temporal lobes, and infarcts involving the perforating artery territories in the thalamus and splenium. Multiple posterior circulation infarcts involving different territories are not uncommon (Fig. 7). Underlying arterial dissection or arteriopathies are frequently identified [18]. Although basilar artery strokes can potentially be life threatening, recent pediatric data suggest a much better prognosis in children compared with adults [18, 19]. Death rate for childhood brain-stem infarction is below 10 %, and survivors frequently have good outcomes. In contrast, recurrent ischemic events after posterior circulation strokes have been described in up to 50 % [18].
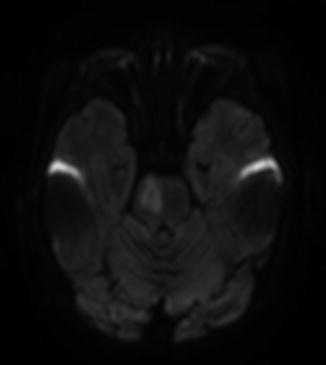
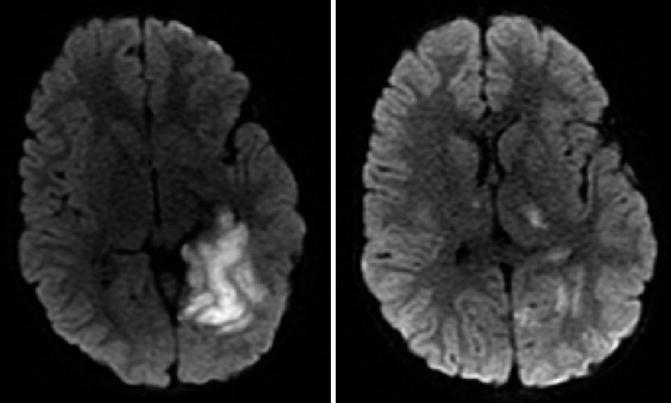

Fig. 6
Three-year-old male patient with acute onset left arm and face weakness. Diffusion-weighted images revealed a right paramedian pontine ischemia

Fig. 7
Five-year-old male with sudden onset headache and unsteady gate. Multifocal diffusion restriction in the posterior circulation
Watershed (Border Zone) Infarcts
In contrast to the majority of CAIS which is due to thrombotic vascular occlusion of one artery, apparently isolated focal ischemic lesions can also be caused by diffuse hypoperfusion. These are usually located in typical watershed or border zones at the anterior, middle, and/or posterior cerebral artery (PCA) junctions. External (cortical) border zone infarcts can be differentiated from those affecting internal border zones (Table 3). In childhood, internal or “deep” border zone infarcts are frequently observed in children with congenital heart disease or after hypoxic–ischemic events (Fig. 8). Differentiating these from cortical embolic strokes is not always straightforward. Although watershed infarcts are typically bilateral, they also can be asymmetric or unilateral.
External (cortical) infarcts |
Frontal cortex (between the anterior and middle cerebral arteries) |
Occipital cortex (between the middle and posterior cerebral arteries) |
Paramedian white matter (between the anterior and middle cerebral arteries) |
Internal (subcortical) infarcts |
Between the lenticulostriate and middle cerebral arteries |
Between the lenticulostriate and anterior cerebral arteries |
Between the Heubner and anterior cerebral arteries |
Between the anterior choroidal and middle cerebral arteries |
Between the anterior choroidal and posterior cerebral arteries |

Fig. 8
Neonate with congenital heart disease and neonatal seizures. Acute MRI revealed multifocal cortical diffusion restrictions. A follow-up MRI 5 days later showed cortical laminar necrosis suggesting a hypoperfusion injury
Internal border zone infarcts, involving the deep white matter, suggest occlusion at the distal internal carotid or proximal anterior cerebral artery (ACA) or MCA frequently occurring with chronic occlusive vasculopathies, including moyamoya (see below).
Hemorrhagic Transformation
Knowledge about hemorrhagic transformation (HT) in CAIS is limited. In a retrospective series of 61 children with AIS, hemorrhagic transformation occurred in 19 (30 %), of whom only two were symptomatic. Increased infarct volume was associated with a higher risk of HT, whereas the risk was low with vasculopathies as the stroke mechanism. Use of anticoagulation and antiplatelet therapy is not a significant risk factor for HT in children [21, 22]. When HT occurs, it is usually ECASS Grade 1 (some of which may actually be laminar necrosis) or Grade 2 and usually asymptomatic [22].
With any episode of neurological deterioration early after stroke, intracranial hemorrhage needs to be considered and emergent neuroimaging is indicated (Table 4).
Table 4
Temporal changes of hematoma on MR imaging (Adapted from Rasalkar and Chu [23] with permission)
Stage and time from hematoma | Hemoglobin stage | Signal intensity on T1 | Signal intensity on T2 |
|---|---|---|---|
Hyperacute (<6 h) | Oxy-Hb | Gray | White |
Acute (6 h–3 days) | Deoxy-Hb | Gray | Black |
Early subacute (3–7 days) | Intracellular meth-Hb | White | Black |
Late subacute (1–4 weeks) | Extracellular meth-Hb | White | White |
Early chronic (>4 weeks) | Extracellular meth-Hb with hemosiderin rim | White | White with black rim |
Late chronic (months to years) | Hemosiderin | Black | Black |
Malignant MCA Infarction
Malignant MCA infarctions in the pediatric population may not be as rare as previously thought. In a recent multicenter hospital-based study, malignant MCA infarctions were identified in 1.3 % of more than 700 children treated for ischemic stroke [24]. However, in another smaller population-based study, 12.5 % (2 out of 16) of all pediatric ischemic strokes over two 1-year periods developed malignant MCA infarctions [25].
Without decompressive surgery, death is frequent. Outcome data in children undergoing decompressive surgery however remains scarce, and specific eligibility criteria for hemicraniectomy in the pediatric population have not yet been defined. However, available data suggest that pediatric survivors of malignant MCA infarction and hemicraniectomy may have a more favorable functional outcome than those without surgery [24]. Therefore, identification of predictors of a malignant course of the MCA in the pediatric population would be of great importance.
In adults involvement of more than 50 % of the MCA territory was found to be a reliable predictor. Preliminary pediatric data suggest that a high pediatric ASPECT score (Fig. 9) and a high pediatric NIHSS are predictive of malignant MCA. Large infarcts (>10 % of total brain volume) also predict poorer functional outcome in children. The modified pediatric ASPECT score correlates well with infarct volume (= percent of supratentorial brain volume) in children of different ages with excellent inter-rater reliability and validity. In addition, the modified ASPECTS assigns points to small but important brain structures (e.g., PLIC), not just volumes, thereby incorporating location along with volume in the score. Therefore, this easy to use score seems to be a useful tool for estimating infarct volume [26].
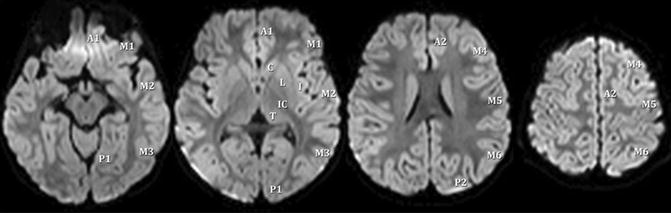

Fig. 9
Scoring areas for the modified pediatric ASPECTS. A1 proximal anterior cerebral artery (ACA) territory, P1 inferior portion of PCA territory, M MCA, C caudate, L lentiform, I insula, IC internal capsule, T thalamus, P2 superior portion of PCA territory, A2 distal ACA territory (From Beslow et al. [26] with permission)
The permeability of the blood–brain barrier as assessed with MRI, PET, and SPECT also predicts a malignant course of MCA infarction. However, these techniques need further prospective evaluation.
Stroke Mimics
In the majority of children referred to tertiary centers with an acute focal neurological deficit, a stroke mimic will be diagnosed including migraine, seizures, Bell palsy, and conversion disorders [27]. Even when stroke is the suspected diagnosis prior to imaging, around 20 % will turn out to be other stroke mimics [28] (Table 5). It can be assumed that the number is even higher in non-tertiary centers. Knowledge about these conditions is therefore of great importance, and establishing the diagnosis requires expertise in both child neurology and pediatric neuroimaging. Careful history taking and examination may help to define a provisional diagnosis. However, MRI will often be required to exclude a stroke with certainty and to establish the diagnosis of AIS in most cases [23]. Many of the conditions mimicking stroke require a specific treatment or further workup.
Migraine |
Conversion disorders |
Seizures |
Postictal paralysis |
ADEM |
Cerebellitis |
Tumor |
PRESa |
Intoxication |
Intracranial abscess |
AVM |
Idiopathic intracranial hypertension |
Periodic hypertensive episodes |
Metabolic stroke |
Subdural empyema |
Moyamoya |
Risk Factors of Arterial Ischemic Strokes
Risk factors associated with CAIS are diverse and differ substantially from adults in whom risk factors act to promote atherosclerosis of the cerebral (or in cardiogenic stroke, the coronary) arteries (Table 6). According to the data from the International Pediatric Stroke Study [10], preexisting risk factors were present in almost 50 % of children presenting with acute ischemic stroke beyond the perinatal period. These consisted mainly of congenital heart disease and other chronic childhood disorders. Following a thorough etiologic evaluation, currently up to 90 % of children will have at least one risk factor identified, with the majority having two or more identifiable factors. Although causality is not always easy to proof, the identification of an underlying etiology is important as it may help to determine the risk of stroke recurrence and lead to a more specific or even causal therapy [5]. Therefore, a systemic diagnostic approach is always warranted. The results of neuroimaging will guide further management.
Table 6
Categorization of childhood arterial ischemic stroke risk factors (International Pediatric Stroke Study) (Mackay et al. [10])
Arteriopathies |
Cardiac disorders |
Chronic systemic disorders |
Prothrombotic states |
Acute systemic disorders |
Chronic head and neck disorders |
Acute head and neck disorders |
Risk factors for artheriosclerosis in adulthood |
The most relevant risk factors of CAIS will be discussed in more detail:
1.
Get Clinical Tree app for offline access

Cerebral arteriopathies
Up to 53 % of children with stroke who undergo vascular imaging have evidence of an underlying cerebral arteriopathy [29, 10]. Since arteriopathies have been shown to be a strong predictor of stroke recurrence, detailed cerebral and cervical vascular imaging for detection and classification of an arteriopathy is crucial in all children with CAIS [5, 16, 17].
Classification of childhood arteriopathies includes six categories:
(A)
Transient cerebral arteriopathy
(B)
Moyamoya
(C)
Fibromuscular dysplasia
(D)
Dissection
(E)
Vasculitis
(F)
Congenital arteriopathies
Among these, the four most commonly encountered will be described below, namely, (A)–(D):
(A)
Transient cerebral arteriopathy
Transient cerebral arteriopathy (TCA) is a nonprogressive, often reversible, unilateral arteriopathy involving the anterior circulation at the circle of Willis. It has been recognized as a leading cause of CAIS [29, 17]. Because the diagnosis of TCA is confirmed by a reversible or stable course on repeated vascular imaging after 6 months, the less specific term focal cerebral arteriopathy (FCA) has been suggested at baseline, when the further course is still unpredictable [30]. However, recently in 79 children with initial imaging showing a unilateral arteriopathy characteristic for TCA, 94 % evolved to definite TCA indicating that the diagnosis can be made with a high degree of confidence on baseline imaging [17]. In the latter study 6 % evolved to other conditions, namely, moyamoya. However, in these children, nearly all had visible moyamoya vessels at onset. More recently it was demonstrated that intracranial dissection can also mimic TCA [31].
TCA has been presumed to be a result of inflammatory mechanisms. Therefore, it has been referred to as nonprogressive childhood primary angiitis of the central nervous system in the rheumatology literature [11]. This forms the rationale for steroid treatment during the initial months after stroke at some centers in combination with antithrombotic and, if post-varicella, with acyclovir treatment. Arterial wall imaging using high-resolution contrast MRI may indeed reveal gadolinium wall enhancement supporting the diagnosis of vasculitis. However, differentiation from normal periarterial enhancement is important. If associated with chicken pox in the preceding 12 months, the term “post-varicella angiopathy” is used [16]. There is now strong epidemiological evidence to support a causal link between a cerebral arteriopathy and varicella zoster infection in the preceding year [32] (Fig. 10).
Common imaging features of TCA are [33]:
1.
Unilateral arteriopathy of the large vessels of the anterior circulation, typically affecting the distal ICA and proximal segments of the MCA and ACA.
2.
Focal or segmental stenosis or occlusion of these vessels on conventional angiography. “Banding” or “striated” appearance is often described on initial CA (Fig. 11). Features of other arteriopathies, such as dissection or moyamoya, are absent.
3.
Dynamic nature during the first weeks and months with repeated imaging often demonstrating worsening of arterial changes.
4.
A monophasic course over the long term, with follow-up imaging after 6 months confirming no further progression, and then either partial or complete resolution of the arteriopathy.
In addition, infarcts are nearly always located in perforator territories within the basal ganglia zone with cortical involvement limited to the overlying insular/temporal cortex. TCA classically affects previously healthy school-aged children. Importantly, the risk of stroke recurrence is at least 18 % and is maximal in the initial few weeks after index stroke. Many patients are left with some degree of residual arterial stenosis. This highlights the importance of serial imaging in these cases.
(B)
Moyamoya disease and moyamoya syndrome
Moyamoya is a rare progressive cerebrovascular disorder characterized by occlusive disease of the terminal internal carotid arteries or the other arteries around the circle of Willis with prominent arterial collateral circulation. The characteristic appearance of a dilated network of lenticulostriate perforating vessels leads to the term “moyamoya” (“puff of smoke” in Japanese). If moyamoya is associated with an underlying condition (e.g., sickle cell disease, Neurofibromatosis Type 1, Down syndrome), it is referred to as “moyamoya syndrome.” Moyamoya disease and moyamoya syndrome occur in children and adults of all ages. Rarely it can present in infancy (Fig. 12). Moyamoya can be bilateral or unilateral.
Common clinical presentations in childhood include headaches, transient ischemic attacks, and AIS. Symptomatic ischemic episodes can often be triggered by crying, blowing, coughing, or hyperventilation. Cognitive decline is another concern. Intracranial hemorrhage, common in adults, is relatively rare in childhood.
Studies from Asia suggest that two-thirds of children will have symptomatic progression at 5 years after diagnosis. The annual stroke risk in asymptomatic pediatric patients was estimated at 3.2 % [34]. Little is known about the natural course of moyamoya in non-Asian children.
After diagnosis it is now widely accepted that all affected children should be referred for surgical evaluation. However, no strict criteria exist to define which child should undergo revascularization surgery. Imaging findings, including cerebrovascular reserve (CVR) studies, clinical symptoms, and ischemic events (TIA, AIS), as well as neuropsychological assessment, should all be taken into consideration.
(a) Imaging of Moyamoya
The diagnosis of moyamoya can be easily made using MRI and MRA (Fig. 13). Besides visualization of unilateral or bilateral stenosis of the terminal internal carotid arteries (ICAs) and the perforator artery collateral network, conventional MRI sequences may demonstrate the so-called ivy sign. This is a sensitive but nonspecific sign for moyamoya describing the presence of high signal intensity at the outer edge of the cortical ribbon on FLAIR sequences, likely reflecting engorged pial vessels. However, artifact-related causes of FLAIR hyperintensity have to be kept in mind, above all in children receiving supplemental oxygen during MRI under anesthesia.
In addition to focal infarction presumably due to thrombotic occlusion in established arterial territories, moyamoya also produces a second, very important mechanism for infarction, namely, hypoperfusion leading to internal or deep watershed infarcts that may be subclinical. The typical imaging pattern of these deep white matter infarcts is a “string of pearls” appearance (Fig. 14). Impaired cerebrovascular autoregulation appears to be an important underlying mechanism for these infarcts [35]. Functional vascular imaging can directly or indirectly determine inadequate perfusion and cerebrovascular reactivity (CVR). In some centers these techniques are routinely used in patients with moyamoya disease and may assist in treatment decisions and help to predict the success of reperfusion after revascularization surgery.
The gold standard to establish the diagnosis of moyamoya is conventional angiography (Fig. 13). Characteristic angiographic findings include stenosis or occlusion at the distal ICA and/or the origin of the anterior cerebral and middle cerebral arteries on both sides. In addition, moyamoya vessels are characteristic reflecting the collateral vascular networks of the lenticulostriate perforators. CA also helps to visualize the dynamics of the blood flow, collateralization, and areas of cerebral hypoperfusion. Before revascularization it also assists in displaying arteries potentially available for bypass surgery (superficial temporal artery, external carotid artery).
(b) Sickle Cell Disease (SCD) and Moyamoya Syndrome
Strokes affect 7–11 % of children with sickle cell disease. Ischemic strokes predominately affect young patients between the ages of 2 and 5. Hemorrhagic strokes have the highest prevalence in patients between the ages of 20 and 29 years with SCD. Silent infarcts were identified in 21.8 % of children between 6 and 19 years of age with SCD-SS in one study. Further studies have shown an estimated cumulative incidence of silent infarct as high as 37 % in patients with SCD by 14 years of age [36].
How SCD leads to cerebrovascular disease is only partly understood and likely multifactorial. The two favored mechanisms include a progressive internal carotid moyamoya-type arteriopathy and an occlusion of small arteries by sickled cells and related thrombus resulting in randomly distributed multifocal small infarcts.
In about 64 % of children with homozygous SCD, stroke is related to a large-vessel occlusive vasculopathy, usually moyamoya syndrome. The supraclinoid ICA is most affected with less involvement of the proximal middle and anterior cerebral arteries. Progression of this vasculopathy with formation of moyamoya collaterals has been reported in up to 40 % of patients [37]. However, a small percentage of children have clinically apparent strokes without evidence of a large-vessel vasculopathy.
Up to 37 % of all children with sickle cell disease will have areas of signal abnormality on MRI without a history of acute neurological impairment indicating silent ischemic infarction. Such children develop progressive cognitive decline as the lesions accumulate. These lesions are typically small and multiple and follow the distribution of the internal white matter border zones. In serial MRI with MRA, screening for presymptomatic cerebrovascular disease is now standard of care in SCD to detect silent infarcts secondary to small-vessel disease and possible moyamoya.
Transcranial Doppler (TCD) screening to measure flow and detect large-vessel arteriopathy and predict stroke risk is now standard of care of children with SCD based on strong evidence [38]. Flow rates above 200 cm/s predict increased stroke risk, and regular transfusion therapy (target hemoglobin S <20–30 %) significantly reduces the stroke risk in children with elevated Doppler velocities. Additional stroke prevention strategies are currently under evaluation including hydroxyurea and nocturnal oxygen supplementation. Aspirin should be considered, especially in cases with large-vessel vasculopathy and/or recurrent stroke. Revascularization surgery may be successful in SCD-related moyamoya.
(C)




Fibromuscular dysplasia
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree