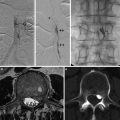
Fig. 1
Three subtypes of cerebral venous and sinus thrombosis (CVST). Based on the affected venous structures, the following subtypes of CVST can be distinguished: dural sinus thrombosis (= thrombotic occlusion of one or more dural sinuses, indicated in red in a), deep cerebral venous thrombosis (= thrombosis of the vein of Galen and its tributaries, indicated in blue in b), and cortical venous thrombosis (= thrombosis of the superficial cortical veins including the anastomotic vein of Labbé indicated in yellow in c). The straight sinus (arrow in b) is included with the deep cerebral veins by most authors. Arrows in (c) indicate the anastomotic vein of Labbé
The most prevalent type of CVST is dural sinus thrombosis (or sinus thrombosis, SVT), which refers to thrombotic occlusion of one or more dural sinuses. The superior sagittal sinus is most commonly affected, followed by the transverse sinus [2]. Deep cerebral venous thrombosis (DVT) affects the internal cerebral veins, vein of Galen, and/or the basal veins of Rosenthal and their tributaries. The straight sinus is included with the deep cerebral veins by most authors. Involvement of the deep cerebral veins is present in approximately 10 % of all patients with CVST and is often accompanied by sinus thrombosis. Isolated thrombosis of the deep cerebral veins is much less common [4] (Fig. 2).
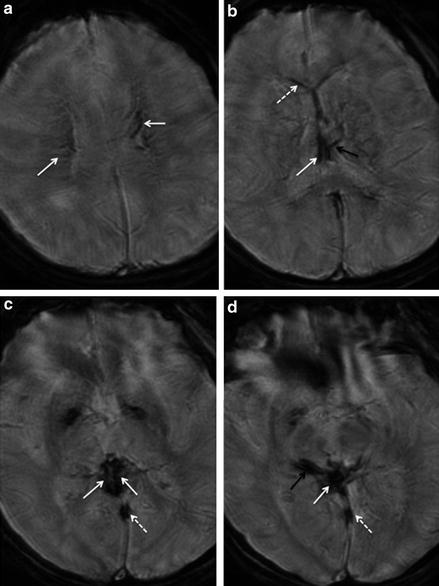

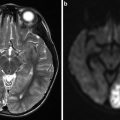
Fig. 2
Deep cerebral venous thrombosis. T2*WI gradient-echo images of a 27-year-old female patient with thrombosis of the internal cerebral veins (normal white arrows in b and c), the vein of Galen (normal white arrow in d), the basal vein of Rosenthal (black arrow in c), and their tributaries (arrows in a, dotted white arrow, and black arrow in d) as well as of the straight sinus (dotted arrows in c and d). Dotted arrow in (b) indicates the right septal vein and black arrow in (b) indicates the left thalamic vein. The venous clot is delineated as linear hypointense signal within the affected veins on T2*WI images (a–d). Note that imaging quality is impaired due to motion artifacts in this patient presenting with impaired consciousness
The term cortical venous thrombosis (CVT) indicates thrombosis of the superficial cortical veins including the anastomotic vein of Labbé (Fig. 1). CVT most commonly affects the frontal cortical veins, followed by the parietal veins. Comparable to isolated deep venous thrombosis, isolated CVT seems to be rather rare and has only been described in case reports and small case series. In the wide majority of cases, concomitant SVT is present, which typically involves the superior sagittal sinus. It is hypothesized that in those combined cases, CVT develops secondary to superior sagittal sinus thrombosis by retrograde spread of thrombotic material from the sinus into the cerebral veins draining into it [5].
Evaluation of the cortical veins is challenging because the cortical veins, unlike the dural sinuses and deep cerebral veins, show considerable intra- and interindividual variations regarding their number, diameter, and anatomic course. This complicates the interpretation of neuroimaging studies, particularly the analysis of angiographic datasets. Within the last decade T2*WI gradient-echo sequences have been found to be very sensitive for the detection of CVT and currently represent the diagnostic gold standard for the diagnosis of this specific subtype of CVST (for details see below) [5–7]. The application of these sequences in the diagnostic work-up of CVST resulted in an increased number of reported CVT cases in recent years. Today it is widely believed that cortical vein involvement is much more common in CVST than previously thought and that isolated cortical vein thrombosis might not be as rare as previously thought but was often missed prior to the wide use of T2*WI sequences in suspected CVST.
Clinical Signs and Symptoms
In contrast to arterial ischemic stroke, CVST usually presents with a more subacute clinical onset with variable, rather unspecific, and heterogeneous symptoms. More or less severe headache constitutes the most common initial symptom of CVST, which is observed in 75–90 % of all patients. Besides, patients often present with other signs of increased intracranial pressure such as dizziness, nausea, and visual disturbances. Additional symptoms of CVST depend basically on the location of the thrombosis, i.e., on the subtype of CVST [1, 8].
Involvement of the deep cerebral veins often results in an altered level of consciousness, which is present in over 70 % of DVT cases [8] (Fig. 2), while cortical venous thrombosis frequently presents with focal or generalized seizures or focal neurological deficits as, e.g., hemiparesis, aphasia, or hemianopsia. In cases with isolated thrombosis of the deep or cortical veins, symptoms of increased intracranial pressure may be absent [9]. Other important factors that determine clinical presentation are patient age, the presence of concomitant parenchymal changes, and the interval from symptom onset to diagnosis [10, 11].
Laboratory Findings
Although normal D-dimer levels have a high negative predictive value for suspected CVST, false-negative results can be observed, particularly in patients with isolated thrombosis of the deep cerebral veins. It has been shown that D-dimer levels may be normal in up to 25 % of cases, presumably due to the relatively small volume of thrombotic material [12].
Prognosis and Outcome
The outcome of CVST has improved significantly in recent decades, largely due to improvements in neuroimaging, which enable promptly diagnosis and treatment with intravenous or low-molecular-weight heparin. To date, CVST has a very good prognosis when diagnosed early, with complete recovery achieved in 80 % of patients [13]. However, it must be mentioned that the average time interval from symptom onset to definitive diagnosis is still 7 days, mainly due to the diverse and unspecific clinical presentation of the disease and its delayed or subacute clinical onset, especially in the small subset of patients with normal D-dimer levels [1].
Delayed diagnosis correlates with a poorer clinical outcome in CVST, which is observed in approximately 10–15 % of patients. Patients with deep cerebral venous thrombosis, males, and patients who only present with signs of increased intracranial pressure are particularly prone to delayed diagnosis of CVST [4, 14]. Other risk factors that are associated with a poor outcome include advanced age, secondary intracerebral hemorrhage due to venous congestion, and involvement of the deep cerebral veins or right transverse sinus [4, 14]. In addition, outcome is worse in patients in whom central nervous system infection or an intracerebral tumor is found as predisposing cause of CVST [4]. Although involvement of the deep cerebral veins is one of the most important risk factors or a poor or even fatal course of the disease, it has been shown that even in this specific subtype the outcome has considerably improved within the recent decades. A study on 32 patients with isolated or combined thrombosis of the deep cerebral veins reports a good clinical outcome with a modified Rankin Scale (mRS) of 2 or higher indicating little or no functional disability in 75 % of patients of DVT patients after prompt treatment with intravenous or subcutaneous low-molecular-weight heparin. Twenty-five percent of patients deteriorated with progressing coma, even though most of them received venous endovascular recanalization therapy [12].
Imaging Features of CVST
Direct and Indirect Neuroimaging Signs
Basically, both direct and indirect signs of CVST can be identified on neuroimaging regardless of the site of the thrombosis. In this regard, “direct signs” indicate imaging abnormalities, which are caused directly by the thrombotic material within the affected veins and/or sinus. Direct signs include both “positive” visualization of the thrombus on unenhanced CT or MRI and “negative” visualization of the thrombotic material as a filling defect in digital-subtraction angiography (DSA), CT angiography (CTA), or other contrast-enhanced images or absence of a flow signal in flow-sensitive venous MR angiography (MRA).
On the contrary, the term “indirect signs” refers to brain parenchymal changes developing secondary to the thrombotic occlusion, e.g., venous edema or venous infarction, subarachnoid hemorrhage, and parenchymal hemorrhage due to venous congestion. In contrast to arterial ischemic stroke, edema or infarction caused by CVST does not conform to arterial territories but crosses territory boundaries. Thrombosis of the dural sinus and/or cortical veins commonly results in venous edema or infarction in subcortical white matter or the cerebral cortex. If the deep cerebral veins are affected, edema or venous infarction typically involves the thalamus and basal ganglia [3]. Particularly in cases with bilateral rather symmetric parenchymal signal abnormalities in the thalami and/or basal ganglia, suspicion for deep cerebral venous thrombosis should be high. The presence of secondary intracerebral hemorrhage at initial diagnosis correlates with more severe initial symptoms and with a poorer outcome [4].
Digital-Subtraction Angiography (DSA)
While DSA has once been considered the gold standard for CVST diagnosis, it no longer plays a significant role in the diagnosis of this disease to date but has been largely replaced by noninvasive imaging modalities, i.e., CTA and MRA. However, due to its higher spatial and temporal resolution, DSA is still superior to MRA and CTA with regard to dynamic information and can yield important additional information particularly in collateral venous drainage. This might justify its diagnostic use in difficult cases.
Some authors hypothesize that DSA might yield a higher sensitivity for cortical venous thrombosis compared to noninvasive imaging modalities, yet systematic data to support this hypothesis is not available. In patients with isolated cortical vein thrombosis, DSA may show an “absent” cortical vein and/or a partially opacified vein with an abrupt cutoff. However, as mentioned above, the value of these direct signs of CVST is considerably limited by the large interindividual variations regarding both the number and anatomic location of the cortical veins. Therefore, diagnosis of cortical venous thrombosis based on DSA mainly relies on indirect signs. The latter include dilated cortical veins adjacent to the site of thrombosis (“corkscrew vessels”), which indicate collateral venous drainage, evidence of a focal delay in venous drainage, as well as signs of venous congestion in the brain parenchyma drained by the occluded vein. The limitations of DSA for the evaluation of cortical veins likewise apply to noninvasive angiographic techniques including CTA and MRA.
Besides its role as an additional diagnostic measure in difficult cases, DSA also is of potential value as a therapeutic tool in selected patients, which are considered for interventional treatment such as local intra-arterial thrombolysis or mechanical recanalization [13]. Although these invasive treatment options have been shown to be effective in single cases and small case series [15, 16], systematic data on their value in CVST are lacking, and they are currently widely regarded as last therapeutic option in otherwise devastating cases.
Magnetic Resonance Imaging
Today MRI including MR angiography is commonly regarded the diagnostic gold standard in suspected CVST although CTA is increasingly reported to be equivalent to MR angiography (see below) [1, 13].
Direct Signs of CVST on MRI
On MRI, CVST presents with rather complex MRI sequence-dependent signal abnormalities, which also vary significantly between the different stages of the disease. Depending on the age of the thrombotic material, an acute, subacute, and chronic stage of CVST can be distinguished which all present with different signal characteristics which are indicated in Table 1. On the contrary, the location of the thrombus, i.e., the subtype of CVST, plays a minor role, as the signal characteristics of the thrombotic material are similar regardless of whether the dural sinuses, the deep cerebral veins, or the cortical veins are involved [3]. Awareness of the significant time- and sequence-dependent variations of thrombus signal intensity is essential for the correct interpretation of MRI datasets in patients with suspected CVST, which requires a great deal of experience in order to avoid diagnostic and technical pitfalls.
Table 1
Stage- and sequence-dependent variability of clot signal characteristics (Modified from Linn and Brückmann [3])
MRI sequence | Signal intensity | Acute stage (0–5 days)a | Subacute stage (6–15 days)a | Chronic stage (>15 days)a |
|---|---|---|---|---|
T1w | Hyperintense | 30 % | 71 % | 39 % |
Isointense | 68 % | 29 % | 54 % | |
Hypointense | 2 % | 0 % | 7 % | |
T2w, PDw, FLAIR | Hyperintense | 25 % | 52 % | 43 % |
Isointense | 10 % | 32 % | 45 % | |
Hypointense | 65 % | 16 % | 12 % |
Spin-Echo T1-, T2-, Proton-Density-, and Fluid-Attenuated Inversion Recovery (FLAIR)-Weighted Sequences
In the acute phase of CVST (days 0–5 from symptom onset), the thrombotic material typically presents with low signal intensity on T2-WI and a relative isointense signal on T1-WI spin-echo sequences (Fig. 3). As these signal characteristics very much resemble those of regular flow voids in patent venous structures, thrombus detection early in the course of the disease is hampered on these sequences. This fact significantly limits the sensitivity of spin-echo MR sequences, especially during the initial days after symptom onset.
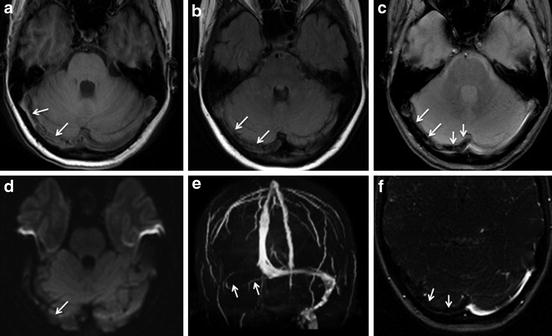

Fig. 3
MRI findings in the acute phase of sinus thrombosis. A 35-year-old female patient with thrombosis of the right transverse sinus. 3-T MRI was performed 3 days after symptom onset. The venous clot (arrows) is partially iso- and partially hyperintense on T1-WI imaging (a), iso- to slightly hypointense on FLAIR sequence (b), and profoundly hypointense on T2*WI imaging (c). It shows no hyperintense signal on diffusion-weighted imaging (d) and delineates as lack of regular flow signal on maximum intensity projection (MIP) reconstruction (e) and source images (f) of venous time-of-flight MR angiography
During the subacute phase (days 6–15), the venous clot becomes increasingly hyperintense in both T2- and T1-WI spin-echo sequences [17] (Fig. 4).
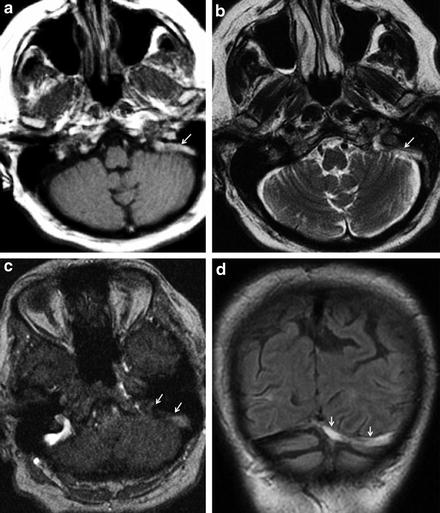

Fig. 4
MRI findings in the subacute phase of sinus thrombosis. A 76-year-old female patient with thrombosis of the left transverse and sigmoid sinuses. 1.5-T MRI was performed 2 weeks after symptom onset. The venous clot (arrows) is hyperintense on T1-WI (a), T2-WI (b), and FLAIR imaging (d) and delineates as lack of regular flow signal on the source image of venous MR angiography (c)
Although these signal patterns are found in the majority of cases, thrombotic signal intensity might vary considerably in the individual case (Table 1). On FLAIR and proton-density sequences, signal characteristics of the thrombotic material are very similar to those found on T2-WI spin-echo sequences [5] (Figs. 3 and 4).
One has to be aware of several potential sources of artifacts and pitfalls on MRI. As an example, a hyperintense signal from normal venous flow might mimic a thrombus on T1-WI spin-echo sequences, if those are acquired without adequate flow compensation. Its proneness to artifacts constitutes a slight disadvantage of MRI compared to CT with CTA and requires expert knowledge to avoid misinterpretation in suspected CVST [3, 17].
Diffusion-Weighted Imaging (DWI)
Venous clot might present with a hyperintense signal on B1000 images of diffusion-weighted MR sequences and with a correspondingly decreased apparent diffusion coefficient (ADC) on ADC maps, which indicates restricted diffusion within the thrombus itself (Fig. 5).
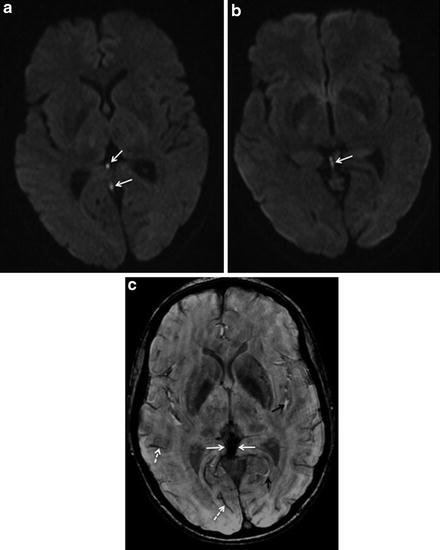

Fig. 5
Hyperintense signal of venous clot on diffusion-weighted imaging (DWI). A 27-year-old female patient with deep cerebral venous thrombosis. DWI images (a, b) performed within the acute phase of the disease show a hyperintense signal of the venous clot (arrows in a and b). On susceptibility-weighted imaging (SWI, c), the thrombotic material is profoundly hypointense (normal white arrows in c). Note also the hypointense normal flow signal in regular veins due to the deoxyhemoglobin fraction of venous blood (e.g., dotted white arrows in c). In contrast, regular arteries with oxyhemoglobin are delineated as hyperintense signal on this sequence (e.g., black arrows in c)
The sensitivity of this sign ranges from approximately 5 % to 40 % in different studies [5, 18]. It has predominately been observed in the subacute stage of CVST. In this stage, the thrombus is typically also hyperintense on T2-, T1-, and FLAIR-weighted images and thus can usually be easily identified without additional information provided by DWI. However, there is initial evidence that thrombus signal characteristics on DWI might serve as a prognostic factor in CVST. In a pilot study by Favrole et al., the presence of restricted diffusion in the clot was associated with low rates of recanalization [19]. The value of this observation should be investigated in further studies.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree