Normal Abdominal and Pelvic Anatomy
Dennis M. Balfe
Brett Gratz
Christine Peterson
More than ever, a successful radiologist must be a practical applied anatomist. To become effective, the radiologist has had to assimilate the standard anatomic information provided by classical dissections. That information is, however, to an increasing extent, inadequate to explain the dynamic anatomic changes observed in pathologic conditions. Radiologists continue to enrich and modify standard conceptions of important structures in the abdomen and pelvis. Examples of the importance of precise anatomic observations abound in the literature: tiny extensions of the pancreatic ductules within a mass can be the sole differential point in diagnosing tumefactive pancreatitis; thin, nearly invisible, fascial planes determine the distribution of fluid collections within the retroperitoneum. The importance of a thorough understanding of human anatomy has grown exponentially as our ability to image it has been refined.
One of the first principles of radiologic practice is that one must first recognize normal structures, including all of their normal variations, in order to confidently diagnose what is abnormal. Now that imaging technology has advanced to the point that subtle anatomic details can be resolved and displayed, radiologists must learn to recognize them and to understand their behavior in health and disease.
The most pervasive single change to occur in the past decade has been the ability to render volumetric data sets in any user-defined anatomic plane, in “rubber-sheet” curved planes, or as images with a component of three- dimensional information content. Although this ability has been available as a laboratory tool for 20 years, the ability to capture a crisp data set made of isotropic voxels, coupled with the introduction of user-friendly software tools, has made three-dimensional (3D) imaging a clinical reality. Accordingly, although many examples of normal abdominal and pelvic anatomy are still well depicted on axial sections, there are many anatomic regions better displayed in other planes (149) or using volumetric display. Accordingly, this chapter will incorporate multiplanar and volumetric renderings whenever appropriate. The internal structure of abdominal organs will be described, but only briefly; the reader is referred to more detailed discussions of the anatomy of each organ in the chapter dealing with that specific system. In all cases, particular emphasis will be placed on those anatomic details that have practical applications to daily imaging procedures and to those regions in which pathologic processes are likely to occur.
ABDOMINAL ANATOMY
Abdominal Wall
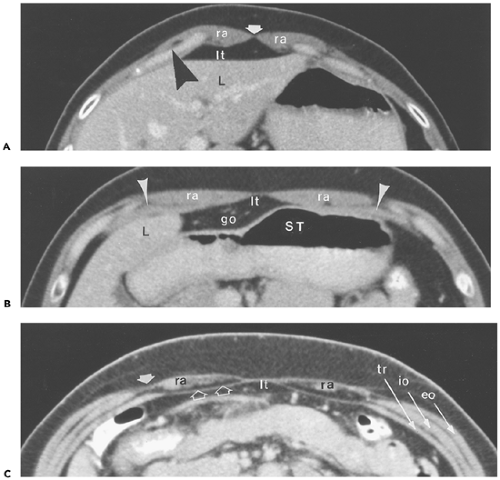
The rectus abdominis muscles compose the anterior aspect of the abdominal wall (47) (Fig. 10-1). They attach to the front of the xiphoid process and to costal cartilages 5 to 7. Extending inferiorly as flat, relatively broad structures, they attach to the pubic symphysis. Above the umbilicus, the rectus muscles are surrounded by a strong sheath formed by the aponeuroses of the three anterolateral muscles (99): the anterior layer is formed by fibers of the external oblique and by a portion of the fibers of the internal oblique. The posterior layer is formed chiefly by fibers from the transverses abdominis muscle, as well as some from the internal oblique. However, about 2 cm below the umbilicus, the posterior portion of the sheath disappears, and fibers of all
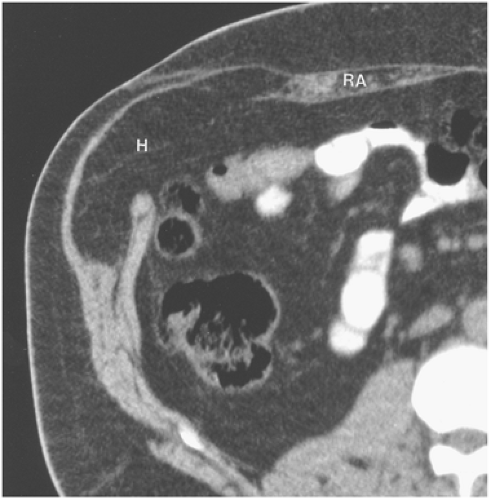
three anterolateral muscle groups pass anterior to the rectus muscle (the zone of anatomic transition is called the arcuate line). This arrangement has clinical significance in that rectus sheath hematomas that occur in the upper abdomen are well confined inside the rectus sheath. Inferior to the arcuate line, however, they can escape into the easily expandable transversalis fascia, and can dissect posteriorly (into the space of Retzius), across the midline, or laterally into the flank (Fig. 10-2).
three anterolateral muscle groups pass anterior to the rectus muscle (the zone of anatomic transition is called the arcuate line). This arrangement has clinical significance in that rectus sheath hematomas that occur in the upper abdomen are well confined inside the rectus sheath. Inferior to the arcuate line, however, they can escape into the easily expandable transversalis fascia, and can dissect posteriorly (into the space of Retzius), across the midline, or laterally into the flank (Fig. 10-2).
Superficial to the rectus muscles, within the subcutaneous fat, are the superficial epigastric veins, which are generally largest on sections through the pelvis (Fig. 10-3). The inferior
epigastric vessels course between the belly of the rectus muscle and the posterior rectus sheath; on sections below the arcuate line, they lie between the rectus muscle and the transversalis fascia. On consecutively inferior sections, the inferior epigastric vessels course laterally within the lateral umbilical fold, which marks the medial aspect of the deep inguinal ring. The vessels also form the lateral boundary of the inguinal triangle (the Hasselbach triangle), the medial boundary of which is the edge of the rectus muscle, and the inferior boundary of which is the pubic bone. Coronal and sagittal images best depict the origin and course of the inferior epigastric artery and vein (see Fig. 10-3).
epigastric vessels course between the belly of the rectus muscle and the posterior rectus sheath; on sections below the arcuate line, they lie between the rectus muscle and the transversalis fascia. On consecutively inferior sections, the inferior epigastric vessels course laterally within the lateral umbilical fold, which marks the medial aspect of the deep inguinal ring. The vessels also form the lateral boundary of the inguinal triangle (the Hasselbach triangle), the medial boundary of which is the edge of the rectus muscle, and the inferior boundary of which is the pubic bone. Coronal and sagittal images best depict the origin and course of the inferior epigastric artery and vein (see Fig. 10-3).
The anterolateral abdominal wall is made up of three paired muscles: from superficial to deep, they are the external oblique, internal oblique, and transverses abdominis. As noted above, the medial aspect of all three muscles is an aponeurosis, which contributes to the formation of the rectus sheath. The lower part of the external oblique aponeurosis forms the inguinal ligament. Magnetic resonance imaging (MRI) has been used effectively to detect strains of the anterolateral musculature (23).
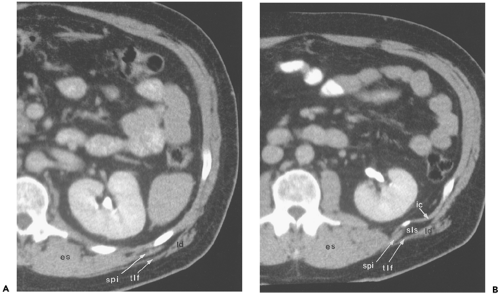
The major muscles of the posterior abdominal wall are, medially, the erector spinae muscles and, laterally the latissimus dorsi (Fig. 10-4). The erector spinae (or sacrospinalis) muscle is really a group of three muscles that cannot be distinguished using standard imaging techniques: from lateral to medial, they are the iliocostalis, the longissimus, and the spinalis. Combined, these muscles are narrow at sacral levels but broad in the thoracolumbar region (54).
The posterolateral surface of the external oblique muscle is covered by the latissimus dorsi, except inferiorly, where the two muscles attach in separate locations to the iliac
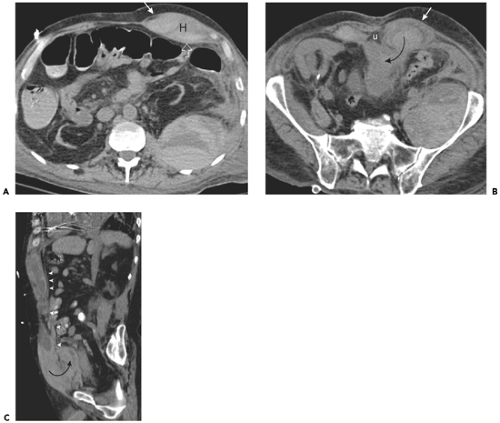
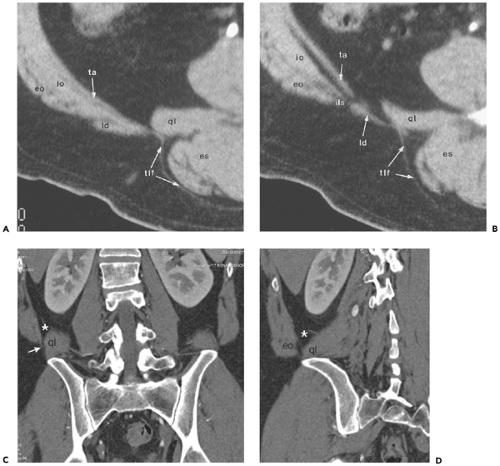
crest. The external oblique muscle attaches anteriorly, whereas the tendon of the latissimus dorsi passes posteriorly. This exposes a small triangle of internal oblique musculature just above the iliac crest. This inferior lumbar triangle (also known as the Petit triangle) (Fig. 10-5) is the site of spontaneous lumbar hernias (11). This area is important to scrutinize in patients with high-speed vehicular trauma, particularly in patients restrained by seat belts (70) (Fig. 10-6).
crest. The external oblique muscle attaches anteriorly, whereas the tendon of the latissimus dorsi passes posteriorly. This exposes a small triangle of internal oblique musculature just above the iliac crest. This inferior lumbar triangle (also known as the Petit triangle) (Fig. 10-5) is the site of spontaneous lumbar hernias (11). This area is important to scrutinize in patients with high-speed vehicular trauma, particularly in patients restrained by seat belts (70) (Fig. 10-6).
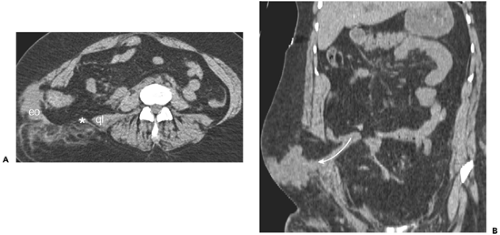
There is, likewise, a relative point of weakness in the aponeuroses of the transversus abdominis and internal oblique muscles just lateral to the rectus abdominis muscle near the level of the arcuate line. This is the classic site for a Spigelian hernia (126). These hernias, when small, are frequently clinically inapparent, because they are confined deep to the strong external oblique muscle (Fig. 10-7).
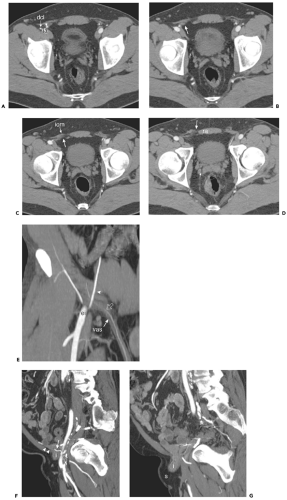
The inguinal canal is the major structure passing out of the abdomen, through the lateral wall musculature. In men, the canal contains the spermatic cord; in women, the round ligament. The deep inguinal ring is a slit-like opening in the transversalis fascia. As its contents pass through the inguinal canal, they are covered by fibers of the internal oblique muscle, which, inferiorly, form the cremaster muscle. Finally, they pass inferomedially through the aponeurotic part of the external oblique at the superficial inguinal ring. Indirect inguinal hernias (which are by far the more common) essentially follow the path of the inguinal contents and therefore begin just lateral to the inferior epigastric vessels (Fig. 10-8). Direct inguinal hernias protrude through defects in the transversalis fascia that comprises the floor of the inguinal canal; they occur medial to the inferior epigastric vessels (77,150).
Computed tomography (CT) is useful in depicting abdominal wall hernias, which, in many cases, are difficult to evaluate clinically because of obesity or scarring from previous surgery. Scans performed with the patient in a lateral decubitus position, or during a Valsalva maneuver, may demonstrate hernias that are not well depicted during standard imaging techniques (1,33,52,65).
Diaphragm, Crura, and Arcuate Ligaments
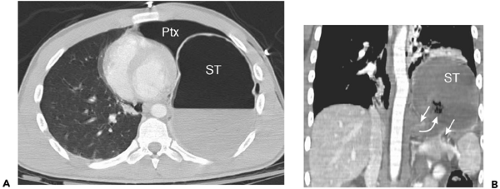
The diaphragm is a large, dome-shaped muscle that incompletely divides the thorax from the abdomen. Its fibers take origin from the sternum anteriorly, from the medial surfaces of the lower ribs anterolaterally, and from the upper lumbar vertebral bodies posteriorly. They insert superomedially on an aponeurosis (the central tendon), the thinnest portion of the diaphragm, which is shaped like an inverted “V” on transaxial sections. The apex of the central tendon lies just anterior to the inferior vena cava; its limbs descend posterolaterally to enclose the vena cava on the right and pass anterior to the esophageal hiatus on the left. It is through the esophageal hiatus that hiatal hernias (as well as ascites, or pancreatic fluid collections) can extend into the posterior mediastinum (84) (Fig. 10-9).
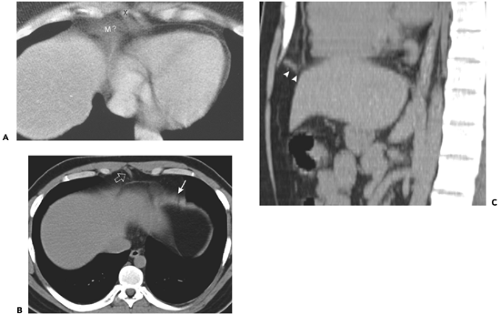
The cross-sectional appearance of the relatively short anterior fibers of the diaphragm depends on the patient’s body habitus and resultant position of the middle leaflet of the diaphragm relative to the sternum. Normally, the central tendon lies 2 to 3 cm cephalic to the xiphoid process, so that cross-sectional images of the diaphragm show a thin soft tissue stripe crossing roughly parallel to the anterior body wall. A more caudally positioned central tendon can produce a confusing image (40), resulting in an anterior pseudomass close to the xiphoid process (Fig. 10-10). In this situation, anterior diaphragmatic fibers course anterior and lateral to their origin on costal cartilage and enclose abdominal fat, forming an arch anterior to liver and heart. This orientation produces fan-like soft tissue stripes projecting from the base of the heart to the anterior body wall. If the central tendon and xiphoid are at the same level, most of the anterior diaphragm will be imaged on a single slice and will appear as a very broad soft tissue band. This portion of the diaphragm is not optimally imaged in the axial plane; these confusing images can easily be resolved using images reformatted in sagittal or coronal planes.
In most patients, the lateral and posterior portions of the diaphragm are perpendicular to the plane of axial section and are displayed as thin soft tissue stripes separating lung parenchyma from abdominal fat. Suspended inspiration, particularly in patients who perform a Valsalva maneuver, allows the muscular slips of the diaphragm to relax and become folded near their costal insertions. They are then displayed as discrete, thick, sometimes nodular soft tissue densities, which may indent the liver, stomach, colon, or spleen, forming accessory fissures or pseudotumors. This is commonly observed in elderly individuals (118) (Figs. 10-11 and 10-12). These infoldings can be distinguished from pathologic nodular densities by noting their continuity with the diaphragm and their separation from abdominal solid and hollow viscera by subdiaphragmatic fat. In problematic cases, repeat imaging in expiration will confirm their identity as diaphragmatic structures (5,117).
The lumbar, or posterior, portion of the diaphragm arises from the crura and the medial and lateral arcuate ligaments. The right and left diaphragmatic crura take origin from the anterolateral surface of the first three right and first two left lumbar vertebral bodies. They unite anteriorly
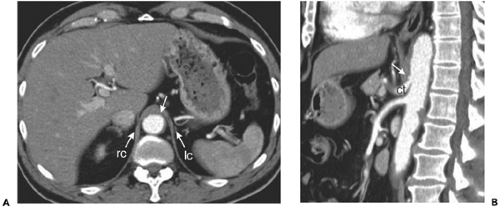
to form the median arcuate ligament, surrounding the aorta immediately cephalic to the celiac trunk (110). This ligament can be imaged as a thin soft tissue stripe crossing the aorta; in some cases, it can produce deformity of the celiac trunk (Fig. 10-13) (134). The right crus is larger and originates lower than the left; it extends to the left of midline. As the crura continue upward, the right crus divides to enclose the esophagus within the esophageal hiatus. As the esophagus enters the stomach, both structures are relatively firmly tethered to the fissure for the ligamantum venosum
by the gastrohepatic ligament. As a result, a portion of the stomach wall runs in a transverse plane, and therefore appears thicker than the rest of the stomach on transverse sections (28). This “pseudomass” has been noted in 25% to 30% of normal subjects (82,138). At the level of the esophageal hiatus, the fibers of the right crus extend almost directly anteriorly, and the most anterior portion may have a bulbous or nodular appearance that mimics left gastric adenopathy. (Fig. 10-14). The smaller left crus remains apposed to the aorta at this level. The posterior aspects of both crura lie against the pleural spaces.
to form the median arcuate ligament, surrounding the aorta immediately cephalic to the celiac trunk (110). This ligament can be imaged as a thin soft tissue stripe crossing the aorta; in some cases, it can produce deformity of the celiac trunk (Fig. 10-13) (134). The right crus is larger and originates lower than the left; it extends to the left of midline. As the crura continue upward, the right crus divides to enclose the esophagus within the esophageal hiatus. As the esophagus enters the stomach, both structures are relatively firmly tethered to the fissure for the ligamantum venosum
by the gastrohepatic ligament. As a result, a portion of the stomach wall runs in a transverse plane, and therefore appears thicker than the rest of the stomach on transverse sections (28). This “pseudomass” has been noted in 25% to 30% of normal subjects (82,138). At the level of the esophageal hiatus, the fibers of the right crus extend almost directly anteriorly, and the most anterior portion may have a bulbous or nodular appearance that mimics left gastric adenopathy. (Fig. 10-14). The smaller left crus remains apposed to the aorta at this level. The posterior aspects of both crura lie against the pleural spaces.
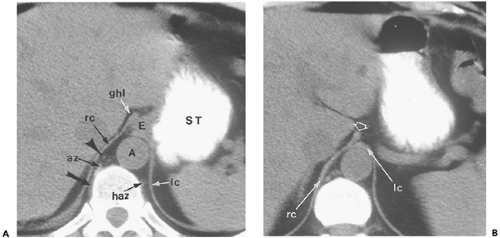
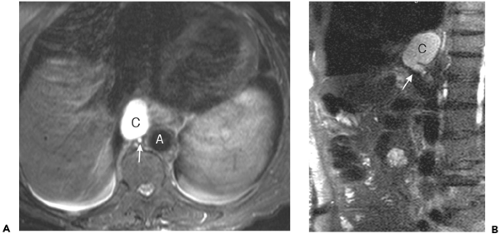
The two crura enclose a retrocrural space anterior to the upper lumbar vertebral bodies (127). The aorta is the major component of this space, but the thoracic duct and azygos/hemiazygos veins also lie within it. The cisterna chyli is very frequently depicted as a bulbous structure of near water attenuation on the right side of the retrocrural space (Fig. 10-15) (46,112). The retrocrural space connects the posterior mediastinum with the abdominal retroperitoneum; pathologic processes in this space are confined on cephalic sections near the diaphragm, but below the median arcuate ligament, may escape anteriorly into the space anterior to the great vessels. Caudal to the median arcuate ligament, the vertebral origins of the diaphragm are seen as fusiform soft tissue densities lying on the lateral surface of the vertebral body anterior to the psoas muscles. On more caudal sections, the crura (most commonly the larger right crus) can be very nodular in appearance, so that they mimic paraaortic lymph nodes (155).
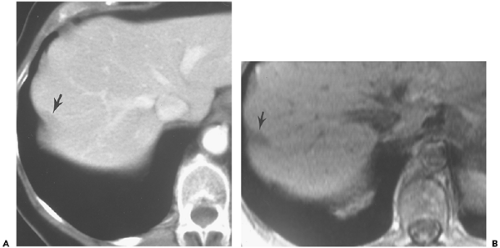
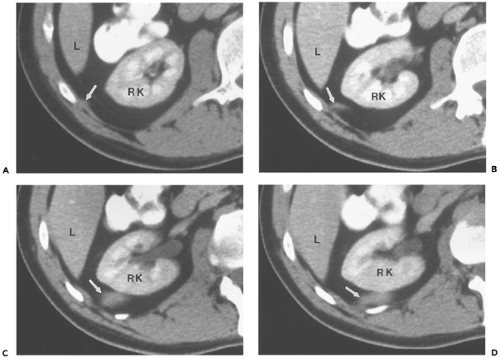
The medial and lateral arcuate ligaments course over the psoas and quadratus lumborum muscles to fuse with the diaphragm (99,109). The medial arcuate ligament extends from the lateral margin of the lumbar spine (L1) vertebral body to the transverse process of L1. The lateral arcuate ligament originates from the transverse process of the L1 vertebral body and inserts on the twelfth rib. In about 5% of normal subjects, the lateral arcuate ligament can appear very nodular (131), and knowledge of its appearance is helpful to avoid confusing it with pathology (Fig. 10-16).
Hernias tend to occur at specific places in the diaphragmatic surface. Anterior (Morgagni) hernias are retrosternal or positioned just lateral to the xiphoid on either side (34); their location may be the result of weakness at the site of penetration of the diaphragm by the superior epigastric vessels. Posterior (Bochdalek) hernias represent incomplete closure of the pleuroperitoneal canal (41). They are depicted on cross-sectional imaging with higher than expected frequency and are at least as likely on the right as
on the left (97) Both can contain abdominal fat or portions of subdiaphragmatic viscera.
on the left (97) Both can contain abdominal fat or portions of subdiaphragmatic viscera.
Cross-sectional imaging has proved useful in evaluating patients with traumatic injuries to the diaphragm (16,17,76,98,100,124). In vehicular trauma, the tears tend to cluster about the junction between the central tendon and the muscular portion of the diaphragm and are much more common on the left. The diagnosis is important, chiefly because of the morbidity of delayed visceral herniation with strangulation; multidetector CT (MDCT) with sagittal and coronal reconstruction has positive and negative predictive values of about 80% (Fig. 10-17).
Intraperitoneal Organs
Embryology
The logic underlying the anatomic distribution of normal intraabdominal viscera and the distribution of pathologic processes arising from them is related to their embryology. Specifically, an understanding of the embryology of the mesenteries and the growth and development of intramesenteric organs provides a basis for understanding the complex nature of the peritoneal spaces in the upper abdomen, the formation of the abdominal ligaments (which play a critical role in defining the course of pathologic processes), and the genesis of the anterior pararenal space. Moreover, the distribution and patterns of extension of fluid collections in the retroperitoneum are explained by the same embryologic processes.
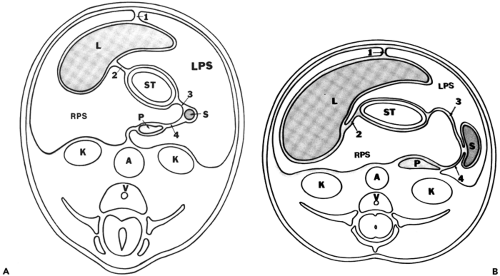
Embryologically, the gut develops from the yolk sac; it is suspended from the anterior and posterior body walls by ventral and dorsal mesenteries, which form the lateral boundaries of the developing alimentary tube. Early in fetal life, important organs develop in the mesenteries of the caudal part of the foregut. The dorsal mesogastrium is the site of the developing spleen and dorsal pancreas, whereas the liver expands within the ventral mesogastrium, along with the ventral pancreas (Fig. 10-18). Major fetal arteries course anteriorly through the dorsal mesenteries from the aorta to supply the developing gut and its newly developing organs. As the liver grows, the ventral mesogastrium thins greatly; the anterior part of it, which in the adult contains the obliterated umbilical vein (ligamentum teres), becomes the falciform ligament. In the adult, this structure is depicted as a midline triangular fat collection containing a fibrous cord that is the remnant of the obliterated umbilical vein. It enters the liver substance at the fissure for the ligamentum teres, forming an incomplete separation between the medial and lateral segments of the left hepatic lobe.
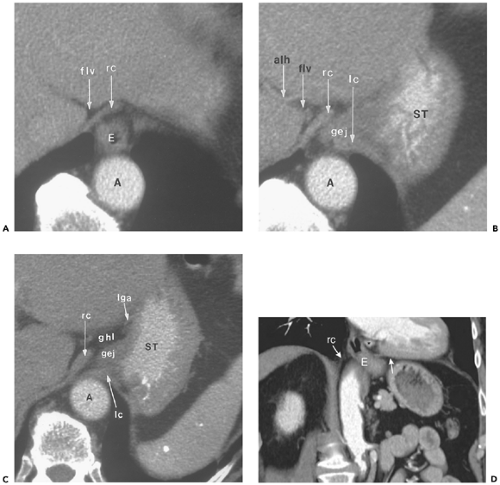
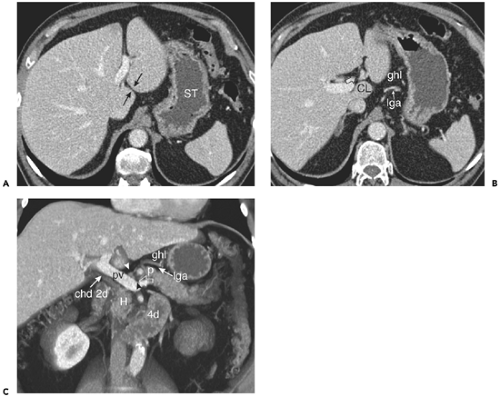
The dorsal part of the ventral mesogastrium (between the liver and the gut) also thins appreciably to form the lesser omentum. In the adult, this structure, with its accompanying fat, stretches between the lesser curvature of the stomach and the fissure for the ligamentum venosum, incompletely separating the caudate lobe from the left lateral segment. Within this gastrohepatic ligament are the left gastric artery and coronary vein, and part of the celiac lymph node chain (6,12) (Fig. 10-19). In some patients, a portion of the caudate lobe (the papillary process) extends between the lesser curve of the stomach and the portal vein and mimics lymphadenopathy (8).
More caudally, the ventral mesoduodenum forms the rest of the lesser omentum, the hepatoduodenal ligament (153,159). This structure encloses the portal vein, hepatic artery, common hepatic/common bile duct, and the hepatic group of celiac lymph nodes.
The spleen grows in the leaves of the dorsal mesogastrium, just posterior to the stomach. The ventral part of this
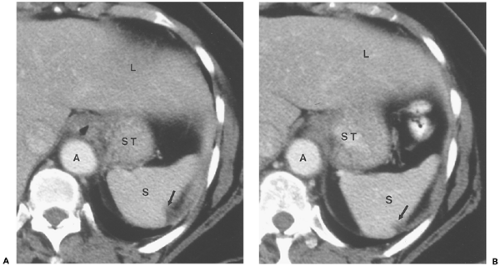
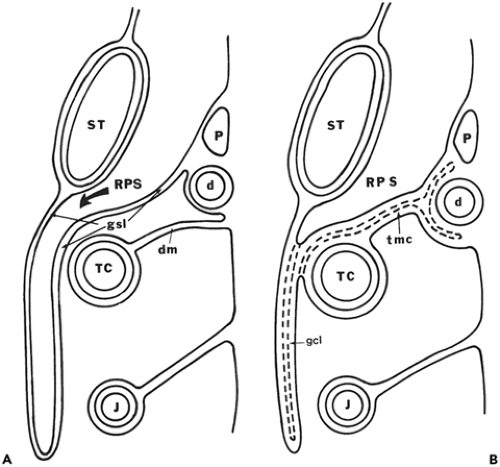
mesentery, the gastrosplenic ligament, ultimately becomes part or all of three very important upper abdominal structures: the greater omentum, the transverse mesocolon, and the gastrosplenic ligament (Fig. 10-20). On sections near the spleen, the ligament is short and is situated posteriorly; it transmits the short gastric vessels behind the inferior recess of the lesser sac (Fig. 10-21). Inferiorly, the peritoneum inside the lesser sac greatly elongates the dorsal mesentery. The apposed redundant surfaces fuse to form the gastrocolic ligament, or greater omentum, which can be recognized by the presence of the gastroepiploic vessels, which arise near the hilus of the spleen (Fig. 10-22) (24). Dilation of the gastroepiploic veins is a very common sign of splenic vein occlusion, frequently a consequence of pancreatitis. A small posterior part of the redundant dorsal mesentery fuses with the dorsal mesentery of the transverse colon to create the multi-layered transverse mesocolon. The root of this structure lies posterior and inferior to the lesser sac and can also be identified by its vessels: the middle colic artery and vein (21) (Fig. 10-23).
mesentery, the gastrosplenic ligament, ultimately becomes part or all of three very important upper abdominal structures: the greater omentum, the transverse mesocolon, and the gastrosplenic ligament (Fig. 10-20). On sections near the spleen, the ligament is short and is situated posteriorly; it transmits the short gastric vessels behind the inferior recess of the lesser sac (Fig. 10-21). Inferiorly, the peritoneum inside the lesser sac greatly elongates the dorsal mesentery. The apposed redundant surfaces fuse to form the gastrocolic ligament, or greater omentum, which can be recognized by the presence of the gastroepiploic vessels, which arise near the hilus of the spleen (Fig. 10-22) (24). Dilation of the gastroepiploic veins is a very common sign of splenic vein occlusion, frequently a consequence of pancreatitis. A small posterior part of the redundant dorsal mesentery fuses with the dorsal mesentery of the transverse colon to create the multi-layered transverse mesocolon. The root of this structure lies posterior and inferior to the lesser sac and can also be identified by its vessels: the middle colic artery and vein (21) (Fig. 10-23).
The dorsal segment of the dorsal mesogastrium extends posteriorly from the spleen to enclose the pancreatic tail and splenic vessels, and then fuses with the posterior body wall, joining with the fascia anterior to the left kidney. This
forms the splenorenal ligament (failure of this fusion process leads to the rare clinical condition of “wandering spleen”) (3). Because of this same fusion, part of the posteroinferior surface of the spleen (adjacent to the upper pole of the left kidney) is not peritonealized. The splenic bare area limits the distribution of fluid in the perisplenic space (144) (Fig. 10-24).
forms the splenorenal ligament (failure of this fusion process leads to the rare clinical condition of “wandering spleen”) (3). Because of this same fusion, part of the posteroinferior surface of the spleen (adjacent to the upper pole of the left kidney) is not peritonealized. The splenic bare area limits the distribution of fluid in the perisplenic space (144) (Fig. 10-24).
The ventral mesentery of the small intestine and colon distal to the midduodenum resorbs and allows free communication of the right with the left peritoneal spaces. The dorsal mesentery of the small intestine simply grows to keep pace with the rapid growth of the small bowel it supports. The dorsal mesentery of the colon, however, has a role to play in forming both the upper peritoneal spaces and the retroperitoneal anterior pararenal space (Fig. 10-25). Initially, the colon is a straight tube lying parallel to the long axis of the fetus and supplied by the superior mesenteric artery via its dorsal mesentery. With time, the colon elongates and eventually, with the small intestine, herniates through the umbilical defect into the extraembryonic coelomic cavity.
When the colon returns to the abdominal cavity, it undergoes rotation (74,94). Its distal part, which will become the descending colon in the adult, maintains the same orientation parallel to the long axis of the fetus. The midportion of the colon rotates 90 degrees (in a counterclockwise direction, as viewed looking down on the fetus) to lie perpendicular to the long axis. The proximal colon, which will become the cecum and ascending portions, undergoes yet another 90-degree rotation in the same counterclockwise mode, so that the original left side of its dorsal mesentery now points to the right side of the abdomen. Finally, the descending colon and its dorsal mesentery rotate (in a clockwise direction, as viewed looking from fetal foot to fetal head), so that its left side fuses to the anterior fascial lining
of the left kidney. In a similar fashion, the ascending colon and its mesentery rotate in a counterclockwise mode, fusing to the anterior fascial lining of the right kidney. The transverse colon does not fuse to the body wall; instead, it “flops” inferiorly, so that the left side of its dorsal mesentery faces anteriorly. It is this surface that fuses to the posterior surface of the gastrosplenic ligament (described previously) to form the transverse mesocolon. Note that as a result of these rotations, the left surface of the dorsal colonic mesentery fuses with the left anterior renal fascia, the gastrosplenic mesentery, and the right anterior renal fascia. This forms an intercommunicating system of planes through which pathologic fluid collections (notably pancreatitis) can be distributed throughout the abdomen (2,48).
of the left kidney. In a similar fashion, the ascending colon and its mesentery rotate in a counterclockwise mode, fusing to the anterior fascial lining of the right kidney. The transverse colon does not fuse to the body wall; instead, it “flops” inferiorly, so that the left side of its dorsal mesentery faces anteriorly. It is this surface that fuses to the posterior surface of the gastrosplenic ligament (described previously) to form the transverse mesocolon. Note that as a result of these rotations, the left surface of the dorsal colonic mesentery fuses with the left anterior renal fascia, the gastrosplenic mesentery, and the right anterior renal fascia. This forms an intercommunicating system of planes through which pathologic fluid collections (notably pancreatitis) can be distributed throughout the abdomen (2,48).
Peritoneal Spaces
Complex rotation and fusion of mesenteric structures alter the peritoneal anatomy; growth of intramesenteric organs, especially the liver, further distorts its appearance. A schematic overview of the distribution of peritoneal spaces in the adult is shown in Fig. 10-26.
The left peritoneal space in the adult can be divided into four compartments (all of which intercommunicate): two perihepatic spaces, anterior and posterior; and two subphrenic spaces, anterior and posterior (Fig. 10-27). The left anterior perihepatic space is confined on the right by the falciform ligament. The left posterior perihepatic space follows the undersurface of the lateral segment of the left hepatic lobe, and extends deep into the fissure for the ligamentum venosum (7,91,122,147). The left anterior subphrenic space is the continuation of the anterior perihepatic space (53). It courses over the gastric fundus just posterior to the anterior leaf of the left hemidiaphragm. Large collections in the left peritoneal space can extend superiorly through the diaphragmatic hiatus into the mediastinum (45), or posterolaterally into the posterior subphrenic (perisplenic) space. This space covers the superior and inferolateral surfaces of the spleen,
but is confined medially by the splenorenal ligament and the bare area of the spleen. Below the spleen, the phrenicocolic ligament separates the perisplenic space from the rest of the peritoneal cavity (Fig. 10-28).
but is confined medially by the splenorenal ligament and the bare area of the spleen. Below the spleen, the phrenicocolic ligament separates the perisplenic space from the rest of the peritoneal cavity (Fig. 10-28).
The right peritoneal space has two major divisions: the perihepatic space and the lesser sac (omental bursa). The right perihepatic space occupies the broad expanse between the anterior and lateral surfaces of the right lobe of the liver and the right hemidiaphragm. It is confined on the left and anteriorly by the falciform ligament, and posteriorly by the right coronary ligament, which marks the lateral aspect of the bare area of the liver (120) (Fig. 10-29). On successively caudal scans, the laterally placed peritoneal recess extends more and more medial as the bare area becomes smaller; at the level of the upper right renal pole, it turns anterior to occupy a space between the posterior liver surface and the anterior renal fascia (121). This posterior recess is named the hepatorenal fossa or the Morison pouch. On scans below the gallbladder fossa, the perihepatic space completely encircles the right hepatic segments (Fig. 10-30).
The anatomy of the lesser sac attests to its complex embryologic origin. As described previously, it is a part of the right peritoneal space that is displaced as the liver grows to fill the right upper abdomen. The right peritoneal space then moves medially behind the gastrohepatic ligament and stomach and is enfolded by the redundant gastro-splenic ligament. As a result, collections in the lesser sac are difficult to access by percutaneous puncture; that space is completely surrounded by mesenteries and intramesenteric organs (96).
In the adult, two major recesses are present. The superior recess completely encloses the medial surface of the caudate lobe (117) (Fig. 10-31). At the porta hepatis, the superior recess lies just posterior to the portal vein; on more cephalic sections, it lies immediately behind the lesser omentum deep within the fissure for the ligamentum venosum
and follows the caudate lobe surface posteriorly and to the right, extending almost to the inferior vena cava. Near the diaphragm, the posterior part of this space lies adjacent to the right diaphragmatic crus, just inferior to the abdominal segment of the esophagus (4). The inferior recess of the lesser sac (see Fig. 10-29) lies between the stomach and the visceral surface of the spleen; on lower sections, it separates the stomach from the pancreas and the transverse mesocolon (30,66). Part of this space may potentially extend between the leaves of the greater omentum (Fig. 10-32).
and follows the caudate lobe surface posteriorly and to the right, extending almost to the inferior vena cava. Near the diaphragm, the posterior part of this space lies adjacent to the right diaphragmatic crus, just inferior to the abdominal segment of the esophagus (4). The inferior recess of the lesser sac (see Fig. 10-29) lies between the stomach and the visceral surface of the spleen; on lower sections, it separates the stomach from the pancreas and the transverse mesocolon (30,66). Part of this space may potentially extend between the leaves of the greater omentum (Fig. 10-32).
Posterior Recesses of the Peritoneum
The precise distribution of the most posterior recesses of the peritoneal spaces depends on the completeness of fusion between the dorsal mesenteries and the posterior abdominal retroperitoneum. When fusion is incomplete, peritoneum may extend posterior to the renal margin on either side. Because mobile structures in the peritoneal cavity can inhabit any portion of the peritoneal space, it is possible for the colon or spleen to lie in a retrorenal or retrogastric position (59,105). Clearly, this variation may be a considerable hazard to patients undergoing percutaneous renal procedures.
Extraperitoneal Organs, Spaces, and Planes
The extraperitoneal spaces include all structures that lie between the external part of the transversalis fascia and the peritoneal lining. There are two major components to the extraperitoneal spaces: the abdominal retroperitoneum, and the pelvic perivesical space. The latter will be considered in the portion of this chapter that deals with pelvic anatomy.
There is really no consistent definition of the region referred to as the abdominal retroperitoneum. The most commonly accepted definition refers to the retroperitoneum as the fat-filled compartment that lies between the parietal peritoneum and the transversalis fascia. However, imaging observations suggest that the retroperitoneum is not a totally separate compartment, but is continuous with portions of the anterior abdominal wall, with the pelvic perivesical extraperitoneal space (27,43), and with the mesenteries discussed in the section on peritoneum.
The purpose of this section is to incorporate these clinical observations of the distribution of retroperitoneal fluid with the classically described anatomy. Rapidly evolving fluid collections (for example, those seen in severe pancreatitis) are too large to be maintained in their space of origin (29,89). Surprisingly, even aggressive processes take very well-defined routes to escape: they either dissect into an adjacent mesentery (a process known as subperitoneal spread) (106,123) or they evacuate into fascial planes that lie between the retroperitoneal spaces (intrafascial spread). It is the latter that explains the connections observed between the diaphragm superiorly and the extravesical pelvic spaces inferiorly (2,15,48,69,93,103,142,158).
Great Vessel Space
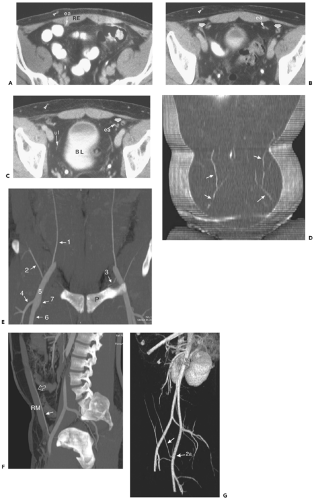
The aorta and its major visceral branches as well as the inferior vena cava and its tributaries course within an ill-defined space anterior to the vertebral bodies (Fig. 10-33). This space is a caudal continuation of the posterior mediastinum, but no discrete fascial planes confine it, and processes may extend from it to involve other retroperitoneal spaces. Paraaortic and paracaval lymph nodes accompany the entire course of
the abdominal aorta and vena cava. The diaphragmatic crura enclose the upper part of the abdominal aorta, ending in a fibrous arch, the median arcuate ligament, immediately cephalic to the level of the celiac trunk. The origin of the superior mesenteric artery is 1 to 2 cm caudal; the course of this artery parallels the aorta over several centimeters. Approximately 1 to 2 cm more caudal, the renal arteries exit the aorta laterally or posterolaterally to enter the renal hila. The right renal artery passes behind the inferior vena cava (IVC) in its course. Above the aortic bifurcation 1 to 2 cm, the inferior mesenteric artery exits the left anterolateral surface of the aorta and gives off left colic, sigmoid, and superior rectal branches.
the abdominal aorta and vena cava. The diaphragmatic crura enclose the upper part of the abdominal aorta, ending in a fibrous arch, the median arcuate ligament, immediately cephalic to the level of the celiac trunk. The origin of the superior mesenteric artery is 1 to 2 cm caudal; the course of this artery parallels the aorta over several centimeters. Approximately 1 to 2 cm more caudal, the renal arteries exit the aorta laterally or posterolaterally to enter the renal hila. The right renal artery passes behind the inferior vena cava (IVC) in its course. Above the aortic bifurcation 1 to 2 cm, the inferior mesenteric artery exits the left anterolateral surface of the aorta and gives off left colic, sigmoid, and superior rectal branches.
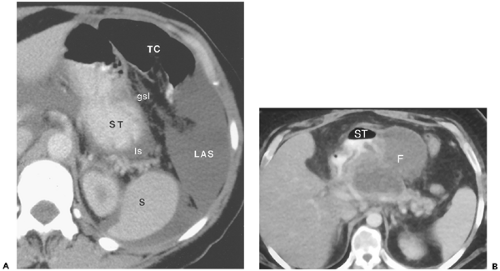
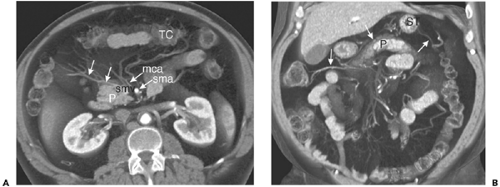 Figure 10-23 The transverse mesocolon. A: Volumetric axial reformatted image through the midabdomen shows the middle colic artery (mca) arising from the superior mesenteric artery (sma) and branching within the transverse mesocolon to supply the transverse colon (TC). Tributaries of the middle colic vein (arrows) here drain directly into the superior mesenteric vein (smv). P, pancreatic head and uncinate process. B: Coronal reformatted image in the same patient shows the transverse mesocolon spanning the entire upper abdomen. The middle colic veins (arrows) mark the position of the mesocolon, which lies immediately superior to the pancreas (P) and inferior to the stomach (ST).
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|