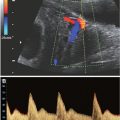
FIGURE 4.1: The mechanical PR or AV interval corresponds to the ECG measurement of the PR interval, the time for the electrical impulse to travel from atrium to ventricle. A pulse waved Doppler is placed to simultaneously sample mitral inflow and aortic outflow in the four-chamber view. The mitral inflow during diastole consists of two phases, E or passive filling and A or active atrial contraction. Ao indicates aortic outflow during ventricular systole. The PR interval is measured from the beginning of the mitral A wave to the beginning of the aortic ejection phase. Normative data for 2 and 3 standard deviations exist in the literature.
Medication Exposure
Fetal exposure to many different maternal medications has been shown to increase the risk of CHD in the fetus. This is dependent on the potential teratogenicity of the substance, when during gestation the fetus is exposed, and how likely it is to cross the placenta in significant quantities. Maternal medications that have been shown to lead to an increased risk of CHD in the fetus include anticonvulsants such as carbamazepine,22 lithium (though more recent literature has suggested that the relative risk is not as high as initially thought),23,24 retinoic acid,25 antihypertensives such as ACE inhibitors,26 selective serotonin reuptake inhibitors,27 and nonsteroidal anti-inflammatory drugs.28
Metabolic Diseases
Uncontrolled phenylketonuria may lead to an increased risk of CHD in the fetus.29,30 Phenylketonuria, with elevated phenylalanine level >10 mg per dL may carry up to 12% to 14% risk of CHD for the fetus.
Viral Infections
Maternal rubella,31 parvovirus, or other infections, including coxsackievirus,32 adenovirus, and cytomegalovirus, can have adverse effects on fetal myocardial function. HIV is not associated with structural CHD23; however, fetuses of HIV-infected women may have alterations of left ventricular function.33 Parvovirus infection is a common cause of nonimmune hydrops fetalis in the fetus. It can cause direct myocardial damage, with reports of conduction system disease,34 or fetal anemia with secondary congestive heart failure. Cardiovascular changes can include chamber dilation, ventricular thickening, and valvular regurgitation.
Assisted Reproductive Technology
An increasingly common indication for fetal echocardiography is the use of assisted reproductive technologies such as in vitro fertilization (IVF), which may or may not be combined with intracytoplasmic sperm injection (ICSI). It is difficult to accurately assess the increase in risk imposed by IVF and/or ICSI on the development of CHD in the fetus, as its use is often more common in mothers of advanced parental age and also leads to an increased risk of monozygous twins, both of which can slightly increase the incidence of CHD. Regardless, the risk of CHD in infants conceived through such assisted reproductive technologies is considered increased and is an indication for fetal echocardiography.35–37
Family History
Another common indication for fetal cardiac evaluation is a family history of CHD. The incidence of recurrence in subsequent family members can vary depending on what the relationship of the fetus to the proband is, and what the nature of the CHD is. The highest risk of recurrence occurs when CHD is present in the mother of the fetus, and fetal echocardiography is indicated in all such cases.38,39 The increase in risk overall is 3% to 7%, but is lesion-dependent, with the most pronounced increase for lesions of atrioventricular septal defect (10% to 14%) and aortic stenosis (13% to 18%). There is also an increased risk of CHD in the fetuses of affected fathers; though the risk of 2% to 3% is not as significant, fetal echocardiography remains reasonable. The recurrence risk for siblings is approximately 3%, though higher for hypoplastic left heart syndrome. The risk to the fetus increases as the number of previously affected siblings increases, and it is not unusual for multiple fetuses of the same parents to be affected with heterotaxy syndrome or aortic valve stenosis.39 Although fewer data are available for the incidence of recurrence in second-degree relatives of affected patients, the risk is thought to be somewhat above that of the general population, but not significant enough to require routine screening in these fetuses.38
Fetal Factors
Multiple factors relating to the fetus itself can be indications for fetal echocardiography.
Suspected Fetal Cardiac Disease
The indication for fetal echocardiography yielding the greatest likelihood of CHD is an abnormal four-chamber view on obstetrical ultrasound. The detection rate of the four-chamber view for significant CHD in the fetus is over 40%.40,41 When outflow tract views are included in the obstetrical screening examination, this number increases to over 50%.40
Suspected Fetal Arrhythmia
All fetuses thought to have an abnormal cardiac rhythm, whether it be tachycardia, bradycardia, or an irregular rhythm, should have a fetal echocardiogram to better assess the specific rhythm abnormality and to diagnose any associated structural heart disease. This can help both in guiding therapy and in evaluating for the underlying cause of the arrhythmia.
Chromosomal Abnormalities
Previously diagnosed or suspected chromosomal abnormalities that are associated with CHD are a common indication for referral for fetal echocardiography. The incidence of detecting CHD varies with the specific chromosomal abnormality in the fetus.42 Commonly referred chromosomal abnormalities include Trisomy 21, 18, or 13, DiGeorge syndrome, and Turner syndrome.
Extracardiac Abnormalities
Even in a genetically normal fetus, extracardiac abnormalities may warrant an echocardiogram. Some abnormalities that are highly associated with CHD in the affected fetus include omphalocele, congenital diaphragmatic hernia, genitourinary abnormalities, and central nervous system abnormalities.43–46
Increased Nuchal Translucency
An increased nuchal translucency found in fetuses between 11 and 14 weeks gestational age has been shown to increase the risk of aneuploidy and other congenital anomalies, including CHD.47,48 The risk of CHD in the fetus rises with increasing NT, even in the absence of known aneuploidy or other genetic abnormality.49
Vascular Malformations
Vascular abnormalities in the fetus that are indications for fetal cardiac evaluation include a single umbilical artery or absence of the ductus venosus. Single umbilical artery is associated with CHD of various types, and in association with renal and vertebral anomalies in the VACTERL syndrome.
Twin Pregnancy
Monochorionic twin gestation carries an overall risk of 2% for CHD. A second indication for fetal echocardiography is to evaluate the heart in fetuses with twin–twin transfusion syndrome. If twin–twin transfusion syndrome is present, structural cardiac anomalies including pulmonary valve stenosis have been described.
Fetal Hydrops
Fetal echocardiography is indicated to rule out structural cardiac disease, arrhythmia, tumor, or vascular anomalies in fetuses with hydrops fetalis. Additionally, scoring systems based on heart size, function, and umbilical Doppler parameters have been created and shown to correlate with outcome.50
FETAL ECHOCARDIOGRAPHY
Timing and Technical Considerations
Timing of fetal echocardiography can vary depending on the indication for referral. The major cardiac structures are present by 9 weeks, and the developmental sequence has been delineated in anatomic specimens using advanced imaging techniques.51 During pregnancy, Doppler assessment of cardiac function can be performed in the early first trimester, even before fetal cardiac development is complete.52 A complete fetal echocardiogram, with the exception of pulmonary vein assessment, is possible at 12 to 16 weeks, with a high level of sensitivity for major CHD.53 However, in general, screening echocardiography for maternal risk factors such as family history or pregestational DM occurs between 18 and 22 weeks gestational age. Follow-up examinations should be performed whenever the initial cardiac evaluation occurs earlier in gestation and/or if any abnormalities are suspected. Once CHD is diagnosed, interval follow-up occurs throughout pregnancy.
Imaging of the fetal heart is limited by the small size of the fetal heart, the rapid heart rate, and the depth of imaging in all cases, and can be additionally compromised by factors such as maternal body habitus and fetal positioning. To optimize imaging, the highest frequency transducer (usually between 4 and 12 MHz) that sufficiently penetrates the maternal abdomen should be used. This may become more difficult later in gestation as the need for increased depth penetration necessitates using a lower frequency transducer. The ultrasound system used should be able to perform two-dimensional (2D) imaging, M-mode, color Doppler, and pulsed-wave Doppler.
Evaluation of the Normal Fetal Heart
Fetal Visceral Situs
The initial step in evaluating the fetal heart is to determine the visceral situs of the fetus, or the laterality of the organs. The normal arrangement of the visceral organs is described as situs solitus, with morphologic right atrium, major hepatic lobe, inferior vena cava, trilobed lung, and short eparterial bronchus on the right side. Situs inversus refers to a reversal in laterality, when these anatomic findings occur on the left side of the fetus. In situs ambiguous, the laterality cannot be determined, and does not fit easily into the solitus or inversus description. Visceral/atrial situs ambiguous is commonly associated with significant CHD, most notably heterotaxy.54 (See Chapter 16)
The first step in determining fetal visceral situs is to determine the exact position of the fetus within the maternal abdomen. By sweeping from maternal foot to head, and from maternal right to left, the exact position of the fetus can be determined. A transverse view of the fetal abdomen and thorax should be obtained, and it can confirm that the fetal heart is pointing to the left side of the chest, as well as the stomach and descending aorta. Typically, the inferior vena cava can be seen on the right side, and the atria into which the inferior vena cava enters is the morphologic right atrium. The inferior vena cava can be interrupted, with azygous continuation to the superior vena cava.
Fetal Cardiac Axis
A transverse view of the chest at the level of the four-chamber view of the heart is found to determine the cardiac axis. A normal cardiac axis lies at approximately 45° from the midline, with the apex of the heart pointing leftward55 (Fig. 4.2). Abnormal cardiac axis, either significantly more or less, can indicate either structural heart disease or other abnormality within the thorax of the fetus such as congenital cystic malformation of the lung or congenital diaphragmatic hernia.
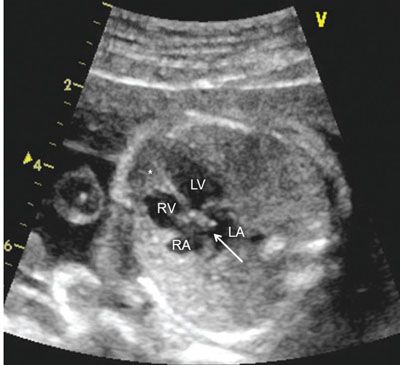
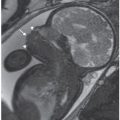
FIGURE 4.2: The four-chamber view of the heart delineates the right atrium (RA), left atrium (LA), right ventricle (RV) and left ventricle (LV). The asterisk indicates the moderator band characteristic of a morphologic right ventricle. The foramen ovale, indicated with an arrow, bows into the left atrium. Note a line drawn through the apex of the left ventricle is at a 45° angle with the midline, which is a normal cardiac axis. The heart lies in the left chest, with apex pointing leftward, and occupies less than one-third of the area of the thorax.
Fetal Cardiac Size
The fetal heart occupies approximately one-third of the area in the thorax. This can be evaluated by tracing the circumference of both the heart and the thorax, and comparing either the area or the circumference. The cardiothoracic area ratio remains fairly consistent throughout gestation and should range between 0.25 and 0.35. Greater ratios may indicate cardiomegaly in the fetus56 and are associated with hydrops fetalis, volume overload lesions such as arteriovenous malformations or sacrococcygeal teratoma, fetal anemia, or myocardial injury from structural heart disease, valvular regurgitation, or arrhythmia.
Fetal Cardiac Position
The position of the heart within the fetal chest is referred to as cardiac position. The normal position is described as levocardia, with the heart located in the left chest (see Fig. 4.2). In dextrocardia, the heart is located in the right chest, and in mesocardia, the heart is located in the midline. The determination of cardiac position is independent of the cardiac axis. For example, with a left-sided congenital diaphragmatic hernia, the heart is positioned in the right chest, but the apex is still pointing leftward. This is known as dextroposition of the heart.
Evaluation of the Fetal Cardiac Chambers
As ultrasound uses 2D images to evaluate the three-dimensional anatomy of the heart, multiple views of all of the fetal cardiac chambers should be obtained.
The Right Atrium
The normal right atrium is located anterior and to the right of the left atrium. Typically, the right atrium receives desaturated blood from the inferior vena cava, superior vena cava, and coronary sinus (Fig. 4.3). At times, the inferior vena cava can be interrupted, but if it enters directly into an atrium, it is typically the morphologic right atrium. The right atrial body is smooth posteriorly and trabeculated anteriorly, with a broad-based right atrial appendage.57 The Eustachian valve, which is located over the opening of the inferior vena cava, directs the highly oxygenated blood from the maternal circulation and, subsequently, the ductus venosus (Fig. 4.4), across the foramen ovale to the left side of the heart, so that the most oxygenated blood supplies the head vessels of the aorta.58
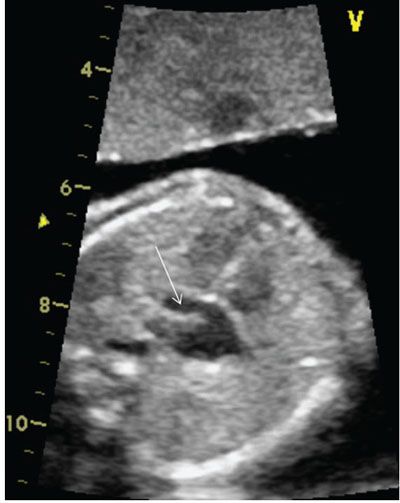
FIGURE 4.3: The coronary sinus is a normal structure located in the posterior–inferior aspect of the heart. Care should be taken not to mistake this normal structure for a primum atrial septal defect. An enlarged coronary sinus, highlighted by the arrow, can be associated with a left superior vena cava, anomalous pulmonary venous connection to the coronary sinus, or with elevated right-sided pressure.

FIGURE 4.4: The ductus venosus should be identified and a pulse wave Doppler performed in a fetal echocardiogram. Here, a normal Doppler flow pattern is seen, with continuous flow entering the fetal heart. Ductus venosus tissue restricts the volume of highly oxygenated maternal blood returning to the fetal heart. Absence of the ductus venosus can cause right heart volume overload. Reversed flow in the ductus venosus is abnormal and can indicate right ventricular diastolic dysfunction or early atrial contraction/arrhythmia, where the atrium contracts against a closed valve.
The atrial septum separates the right and left atria, which is formed by the septum primum and septum secundum. The fossa ovalis is on the right atrial side, flow across the fossa ovalis is typically right to left (Fig. 4.5). Septum primum forms the valve of the foramen ovale, and is located within the left atrium. Atrial septal defects are classified by where they lie within the atrial septum.

FIGURE 4.5: Unrestricted foramen ovale flow is seen crossing from right to left (normal) in this fetus. Restriction of the atrial septum can be seen in CHD as well as as an isolated finding, and careful attention should be paid to the size and direction of the foramen flow. The foramen ovale typically constitutes less than one-third of the atrial septum.
The Left Atrium
The normal left atrium is located posteriorly within the chest cavity and is smooth-walled. It should receive all four pulmonary veins in the normal heart and is smooth-walled throughout. The left atrial appendage is narrow and fingerlike.57 It contains the septum primum or the flap valve of the foramen ovale (see Fig. 4.2).
The Right Ventricle
Located behind the sternum, the right ventricle is the most anterior chamber in the normal heart. It is coarsely trabeculated in contrast to the smooth trabeculations of the left ventricle. It is crescent shaped as opposed to elliptical like the left ventricle. Although the left ventricle is designed to handle the demands of the systemic circulation after birth, in utero it is the right ventricle that pumps against high resistance of the pulmonary circulation. It contains a moderator band (see Fig. 4.2).
Tricuspid Valve
The atrioventricular valve is defined by its ventricle, with the tricuspid valve directing blood into the right ventricle. The tricuspid valve should be slightly more apically displaced within the heart than the mitral valve. The tricuspid valve is comprised of three leaflets—the anterior, posterior, and septal leaflets—which are anchored by the chordae tendineae to the three papillary muscles within the body of the right ventricle. Chordae tendineae from the tricuspid valve attach directly to the septal wall in the right ventricle, a feature that is not found in the left ventricle.
The Left Ventricle
The left ventricle is smooth-walled, and located posterior and leftward of the right ventricle. It is elliptical or bullet shaped and should form the apex of the heart. It does not have a moderator band and has two distinct papillary muscles that should be clearly separated (the anterolateral and posteromedial papillary muscles).
Mitral Valve
The mitral valve is the atrioventricular valve associated with the morphologic left ventricle. The mitral valve has two leaflets—the anterior and posterior leaflets—that attach to the anterolateral and posteromedial papillary muscles, which then insert into the free wall of the left ventricle, without the septal attachments apparent in the morphologic right ventricle.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree