Radioisotope
Half-life (approx.)
81mKr
13 s
99mTc
6 h
131I
8 days
51Cr
1 month
137Cs
30 years
241Am
462 years
226Ra
1,620 years
238U
4.51 × 109 years
Nuclear Imaging Technique
The images are obtained by mapping the distribution of an administered radiopharmaceutical within the body. The radiation is emitted from within the patient and subsequently detected in the imaging device, unlike transmitted through the patient from an external X-ray source (CTs and radiographs). The specific organ function depicted is determined by the biological behavior of the radiopharmaceutical. Conventional imaging with the use of a gamma camera is referred to as planar imaging. More recently, the single photon emission computed tomography (SPECT) has been developed which produces axial slice imaging through the body. SPECT uses a gamma camera to record images at a series of angles around the patient, and the resultant data can be processed using filtered back projection and iterative reconstruction algorithms. SPECT gamma cameras can have one, two, or three camera heads. The more advanced imaging is positron emission tomography (PET) that is also an axial projection acquisition-based technique. PET exploits the positron annihilation process where two 0.51 MeV back-to-back gamma rays are produced. If these gamma rays are detected, their origin will lie on a line joining two of the detectors of the ring of detectors which encircles the patient.
It took more than 40 years for the PET to reach its current state as a clinically useful tool. It is primarily due to the challenge in engineering the electronic components of medical imaging instrument to be merged into the contemporary imaging modality. The major breakthrough in positron imaging heralded with the development of positron camera in 1960 which produce planar images [1]. Later the same year, true transaxial positron tomography utilizing a ring system of detectors was produced by Brookhaven National Laboratory group.
With the advent of advanced reconstruction techniques accompanying CT, the PET scanning took a giant leap ahead. The prototype of modern day positron emission computed tomography was first implemented by Phelps and colleagues in the mid-1970s [2].
More recently, the limited anatomical definition of radionuclide imaging has been addressed to some degree by the development of hybrid imaging techniques in which radionuclide imaging devices are combined with computed tomography in a single imaging system [3]. The resulting images display the functional data obtained from the radionuclide distribution (in color), overlaid on the anatomical information from CT (in gray scale) [3]. As the two image data sets are acquired almost simultaneously using the same imaging device, the two data sets can be co-registered very accurately. Not only do these hybrid systems allow abnormalities seen on radionuclide images to be assigned to precise anatomical structures, but they also enable the morphological appearances of disease processes depicted by CT to be assimilated into the interpretation of the findings on radionuclide images. For example, such combined interpretation can aid the distinction of malignant and inflammatory causes of uptake of the positron-emitting radiopharmaceutical 18F-fluorodeoxyglucose (FDG) [4]. Further advantages of hybrid systems include the use of the CT data to correct radionuclide images for artifacts resulting from attenuation of photons travelling through the body and the ability to incorporate radionuclide image data into CT-based radiotherapy planning systems.
Radiotracer in Human Studies
The radioactivity is generally administered to the patient in the form of a radiopharmaceutical agent or radiotracer. This follows some physiological pathway to accumulate for a short period of time in some specific organ of the body (Table 14.2). A good example is 99mTc-tin colloid which following intravenous injection accumulates mainly in the liver. The substance emits gamma rays, and we can produce an image of its distribution using a nuclear medicine imaging system. This image can tell us the physiological functional information of the liver or localize the diseased sections.
Table 14.2
Specific organ where radiopharmaceutical agent or radiotracer accumulates for a short period of time
Body organ | Radiotracer |
|---|---|
Brain | 99mTc-ceretec |
Thyroid | Na99mTcO4 |
Lung (ventilation) | 133Xe gas |
Lung (perfusion) | 99mTc-MAA |
Liver | 99mTc-tin colloid |
Spleen | 99mTc-damaged red blood cells |
Pancreas | 75Se-selenomethionine |
Kidneys | 99mTc-DMSA |
Nuclear medicine procedures are best served by tracers labeled with a radionuclide that has a physical half-life that is long enough to allow for imaging in a reasonable amount of time, but not so long as to continue to irradiate the patient much beyond that imaging. Thus, radiotracers cannot be stored but must be generated daily for immediate use. To provide for tracers labeled with short-lived radionuclides, generators containing the parent material are constructed to provide an extended source of the daughter; alternatively, the radionuclide may be generated in a medical cyclotron, used to label a tracer, and the tracer shipped to the nuclear medicine department for use. This latter method is generally employed for positron-emitting radionuclides, the exception being rubidium-82, a blood flow PET tracer produced in a generator. The most commonly used radionuclide in nuclear medicine procedures is Tc-99m, and the generation of this radionuclide is from molybdenum-99 (Mo-99)-Tc-99m generator. Discussion on moly-generator is beyond the scope of this book.
Other widely used radionuclide tracers which need to be discussed in detail are the PET radionuclides. The most commonly used radionuclides for PET include fluorine-18 (F-18), carbon-11 (C-11), nitrogen-13 (N-13), and oxygen-15 (O-15). In fact, the successful synthesis of F-18 and the application of 18F-FDG had provided another major drive for the advancement of PET [5, 6].
FDG is the most commonly used tracer for PET but is plagued with false-positivity. For instance, the overall accuracy of FDG-PET in detecting a solitary pulmonary nodule is in the order of 90 %, but false positivity due to granulomatous diseases like tuberculosis leads to incorrect diagnostic categorization due to similar uptake of FDG by affected cells. Tracers that use cellular mechanism for which tumor tissue will be different from that of normal tissue will reduce the incidence of false positivity. One such mechanism that is prominent for tumor tissue is its high cellular proliferation. Tracer targeting this cellular feature will provide an appropriate diagnostic categorization of tumor tissue. F-18 fluoro-deoxy-L-thymidine (FLT) is a promising candidate which gets incorporated in the DNA in a fashion similar to that of natural thymidine in the cell nucleus. The cellular proliferation is earmarked by mitosis which leads to DNA synthesis and hence specific increased uptake of FLT which can be imaged using FLT-PET. On the downside, organs like the liver and bone marrow have high affinity for FLT even under normal conditions due to high cellular turnover. Hence, FLT is of less use in detecting primary or metastatic lesion in these sites.
Cellular Mechanism of Radiotracers
The success of nuclear medicine applications depends on the detection of signals emitted by an injected radionuclide that is concentrated in the pathological tissue under evaluation. For the radionuclide to concentrate in the specific target tissue, it should be tagged to a modified biomolecule for which the cells at the target location have high affinity for specific uptake. The substitution of radionuclide onto the biomolecules will not significantly alter the reaction time or mechanism of the molecule; hence it is taken up as if it is the natural substrate for the cell.
The common cellular mechanisms that are the targets for tracing the specific uptake of radionuclides are:
1.
Glucose utilization of the cell targeting the glycolytic pathway: All cells utilize glucose as a substrate for energy. The glucose is taken up by the cell via GLUT membrane transporter. FDG is a glucose analogue that enters the cells via the same membrane transporters as glucose. Glucose as well as 18F-FDG is phosphorylated by the enzyme hexokinase. In contrast to glucose-6-phosphate, 18F-FDG-6-phosphate is not a substrate for further metabolism in the glycolytic pathway. Therefore, 18F-FDG-6-phosphate is trapped in the cells in proportion to their glycolytic activity [7].
2.
Cellular proliferation mechanism: The proliferation rate of a normal cell is different from that of malignant cells. DNA synthesis is high in rapidly proliferating malignant cells. A carefully fluorinated analogue of a pyrimidine or a purine base will behave in the same manner as natural bases and gets incorporated into the DNA of rapidly proliferating tissues. F-18 fluoro-deoxy-L-thymidine (18F-FLT) is a fluorinated analogue of thymidine. The specificity of FLT is high for malignant tumors that have a high cellular proliferation rate relative to that of the nonmalignant tissue. They yield false-positive results for tumors in organs like liver and bone marrow which innately have high cellular turnover.
3.
Protein synthesis machinery: Malignant tumors characteristically have high protein synthesis in comparison with benign tumors. Specific amino acids labeled with radionuclide will be taken up by malignant tumors for protein synthesis. A high concentration of radiolabeled amino acid analogues in a tumor can be imaged using PET scanner. Examples include 11C-methionine and F-18 fluoroethyltyrosine (18F-FET).
4.
Choline synthesis: Low-grade tumors have less affinity for 18F-FDG and 18F-FLT. Many low-grade tumors are characterized by high choline content. Presumably these cells are also associated with choline transport and involved in sterol metabolism. 18F-fluorocholine, a radiolabeled choline analogue, has provided promising results in some 18F-FDG false-negative cases [8].
5.
Tumor vascularization: Proper vascularization is required for tumor growth and for metastasis. Inadequate vascularization especially in the core of the tumor results in hypoxia and necrosis. Tumor tissue hypoxia could be a good mechanism to target in several solid tumors. 18F-fluoromisonidazole (FMISO) radionuclide tracer is a promising candidate specific for tissue hypoxia in vivo. FMISO in combination with 15O-water perfusion imaging has been used to assess the presence and severity of intratumoral hypoxia, a major determinant of treatment resistance [9]. Another recent addition that targets tissue hypoxia is 18F-fluoroazomycinarabinofuranoside (FAZA) [10].
Radiation Safety
Radiation exposure is a very critical issue which needs to be addressed in all radionuclide studies. If radiation exposure exceeds the permissible limit, it will have effects that may range from trivial to fatal with short-term and long-term sequela-like radiation sickness, vomiting, alopecia, radiation enteritis, GI bleeding, radiation burns, skin carcinoma. On an average, each individual is exposed to a natural background radiation of 3 mSv annually from naturally occurring radioactive materials and cosmic rays from outer space while the largest source of background radiation comes from radon gas. The maximum permissible dose (MPD) per year is for:
Occupationally exposed individuals—50 mSv:
Optimal design goal for restricted area should not exceed 5 mSv/year.
Individuals who are infrequently exposed or in contact with patients receiving radionuclide therapy—5 mSv.
General Public—1 mSv.
Individuals subjected to X-ray security screening—0.25 mSv.
370 MBq of 18F-FDG delivers a dose of 11 mSv to a patient. A patient who has undergone a therapeutic procedure with sealed or unsealed radionuclides may deliver a high radiation dose to people coming in contact with him/her. Hence, a guidance level of 1,000 MBq has been laid as a standard for discharge of patients who had recently undergone radionuclide therapy.
See Table 14.3 for the diagnostic CT effective radiation doses. See Table 14.4 for effective radiation doses for various radionuclide studies.
Table 14.3
Diagnostic CT effective radiation doses
Organ | Radiation dose (mSv) |
|---|---|
Heada,b | 2 |
Chesta,c | 8 |
Abdomena,c | 10 |
Pelvisa,c | 10 |
Table 14.4
Effective radiation dose for various radionuclide studies
Radionuclide study | Radiation dose (mSv) |
|---|---|
Lung ventilation (Xe-133)a | 0.4 |
Lung perfusion (Tc-99m)a | 1.2 |
Kidney (Tc-99m)a | 2.2–2.5 |
Thyroid (Tc-99m)a | 2.6 |
Bone (Tc-99m)b | 4.8 |
Cardiac gated study (Tc-99m)a | 4.2 |
PET/CT whole body (F-18 FDG)c | 7–8 |
Radiation Exposure to the Workers
The total average exposure for a radiology technician or technologist results in an annual dose equivalent of only 1–1.5 mSv, whereas for the nuclear medicine technologist, it is 2–2.5 mSv. The majority of whole-body radiation to the nuclear medicine worker comes from exposure to the dosed patient during imaging.
Radiation Exposure to the Patient
As most nuclear medicine procedures require an injection of radioactive material attached to a tracer, utmost care must be taken to ensure that the correct procedure has been selected to maximize diagnostic suitability, that the radioactivity of the injected dose follows the principles of ALARA (as low as reasonably achievable) or ALARP (as low as reasonably practicable), and that the patient has been given adequate information prior to the procedure and such aftercare details as are appropriate. All nuclear medicine procedures require that precautions be taken when administering radioactive materials to females of child-bearing age.
Good Clinical Practice (GCP) and Good Manufacturing Practice (GMP)
Knowledge and compliance with the good clinical practice (GCP) is essential for everyone involved in clinical research trials for nuclear medicine. Clinical trials should be carried out within the framework of a good clinical practice environment in accordance with international guidelines and regulations as detailed in the Declaration of Helsinki [11, 12]. The International Commission of Radiological Protection and the World Health Organization (WHO) have publications that deal with this issue in clinical research. While GCP should form the backbone to successful nuclear medicine clinical studies, radiopharmaceuticals used in these trials need to be produced according to the good manufacturing practice (GMP) [13].
Radiopharmaceuticals for clinical research purposes must be manufactured in accordance with the basic principles of GMP. Due to their short half-lives, many radiopharmaceuticals are administered to patients shortly after their production, so some elements of the quality control may be retrospective. Therefore, strict adherence to GMP is essential. Special attention should be given to the production area environment and personnel, the two basic requirements of GMP production.
Quality Control
Since lots of confounding parameters exist in nuclear medicine, it is a good practice to stick to certain established standards laid by the regulatory bodies, advisory committee, professional organization, and manufacturer’s guidelines to ensure quality control of the imaging equipment, radiopharmaceutical tracers, procedure, and imaging protocol. Quality control starts at the time of installation and continues throughout the life cycle of the equipment. It is an ongoing process involving measurements and analyses designed to ensure that the performance of a procedure or instrument is within a predefined acceptable range and to keep it operating at peak performance all the time [14]. Therefore, it is a critical component of routine nuclear medicine practice. A detailed explanation on the quality control program is beyond the scope of this book.
The common quality control measures followed routinely in nuclear medicine practice are:
Uniformity calibration.
Spatial linearity.
Energy resolution and peaking.
Pixel size calibration.
Center of rotation correction.
Tomography resolution.
Normalization scan.
Uniformity Calibration
The uniformity in the performance of the crystal detector is important for the diagnostic quality of all images. Any nonuniformity in the performance can be demonstrated by an image called the flood image that is irradiated by a uniform distribution of radioactivity. From the high-count flood image of a particular radionuclide, the uniformity (or sensitivity) correction table is derived for that radionuclide which is essentially the pixel-by-pixel ratio of the calculated mean count per pixel to the actual count per pixel in the flood image. Causes of nonuniformity may be due to cracked crystals because of mechanical trauma or temperature excursion beyond threshold, improper tuning of photopeak of radionuclide with the photopeak energy window of camera, uncoupling of photomultiplier tubes from crystals, corrupted software correction tables, etc. Pixel-by-pixel multiplication of an uncorrected image by the ratio image thus calculated gives an image corrected for the nonuniformity.
Uniformity calibration could be evaluated either intrinsically by a point source of 99mTc placed at about 5 crystal dimensions in the z direction from the center of the uncollimated detector so that a uniform photon flux spreads over the detector (with a count rate of >25,000 cycles/second) or extrinsically by using a sheet source of 57Co placed over the collimated detector (with a count rate of 10–15 million cps). This yields the integral and differential uniformity which expresses the deviation from flood image uniformity.
In current generation nuclear imaging systems, the integrated software solution provides uniformity correction tables that can be easily updated, processed, stored, and automatically applied.
Spatial Resolution
Spatial resolution is the power to discriminate two closely placed point sources distinctly. The overall resolution of a gamma camera is based on the sum of intrinsic spatial resolution of the detector system and the geometric resolution of the collimator. To evaluate the spatial resolution of a gamma camera, a wide variety of test patterns have been developed over the years. The most common test pattern is the four-quadrant bar phantom, which accounts for over 80 % of all resolution patterns used in nuclear medicine. Apart from this, several other test phantoms are available. All these provide a qualitative index of resolution. Correct use of these phantoms requires that all images be compared with a reference image. This reference image can be obtained during acceptance testing or when a quantitative measurement of intrinsic resolution is being performed on the system.
The modulation transfer function (MTF) is an index that exhibits the ability of a gamma camera to yield an image corresponding exactly with the physical distribution of the radionuclide.
Spatial Linearity
Spatial linearity may be affected with nonuniformity due to ill-defined factors like variations in crystal thickness. Spatial linearity correction is done by presenting the camera with an image consisting of a series of parallel straight lines aligned with either the X or Y axis of the camera. The deviation between the true position of each point on the line, as calculated from a best-fit straight line, and the image of the line, is recorded and stored as a correction factor to be applied to subsequently acquire clinical images.
The correction is generally performed on a weekly basis using either intrinsic or extrinsic techniques.
With this basic idea of nuclear physics, imaging, radiopharmaceuticals, and quality monitoring, we will now proceed to organ-based applications of the technique as a problem-solving tool in medicine.
Nuclear Imaging in Oncology
Positron emission tomography (PET) is an imaging technique based on nuclear physics principles that provides in vivo measurements in absolute units of a radioactive tracer. One of the attractive aspects of PET is that the radioactive tracer can be labeled with short-lived radioisotopes of the natural elements of the biochemical constituents of the body (18F-fluoro-2-deoxy-glucose or [18F]-FDG). This provides PET with a unique ability to detect and quantify physiologic and receptor processes in the body, particularly in cancer cells, which is not possible by any other imaging techniques today. Oncologic PET studies now represent almost 90 % of all clinical studies performed in clinical PET centers worldwide [15–19].
Although 18F-FDG is the most commonly used positron-emitting tracer in PET imaging, there are measurement of tissue blood flow, oxygen metabolism, glucose metabolism, amino acid and protein synthesis and nucleic acid metabolism that have all been demonstrated in PET oncology clinical studies using other tracers [20–23]. Labeling of a large array of other compounds including hypoxic markers, amino acids, DNA proliferation markers, and chemotherapy drugs with 11C and 18F has been studied in various clinical trials (Table 14.5) [17, 22, 23].
Table 14.5
Positron-emitting radionuclides used in oncology clinical studies
Radionuclide | Half-life |
|---|---|
15O | 122 s |
13N | 9.97 min |
11C | 20.4 min |
18F | 109.8 min |
124I | 4.17 days |
86Y | 14.7 h |
64Cu | 12.8 h |
The practical and ethical issues associated with PET examination poses difficulty in the conduct of randomized controlled trials; thus, the establishment of diagnostic accuracy and impact on patient management are mainly obtained from clinical practice [18, 24]. There is increasing evidence for the role of PET in staging, monitoring treatment response and biologic characterization of tumors [15–19, 21–23, 25–30].
Brain Tumor
The evaluation of brain tumors with 18F-FDG PET is a well-established oncologic application of PET. Tumor grade can be assessed accurately and noninvasively by 18F-FDG PET, as the rate of glucose (tracer) utilization is directly proportional to the degree of malignancy [31]. This can be used in the planning of biopsies, and in monitoring high-grade recurrence, particularly in patients with low-grade glioma. Increased 18F-FDG uptake is seen in high-grade glial tumors, as well as in primary cerebral lymphomas, pilocytic astrocytomas, and some unusual tumors (e.g., pleomorphic xanthoastrocytoma, low-grade gliomas). Primary brain tumors (e.g., meningiomas) do not usually show increased 18F-FDG uptake except in more aggressive tumors and in postradiation meningiomas.
Secondary cerebral metastases occur in almost 20–40 % of systemic malignancies and may be the initial presentation of malignancy in 16–35 % of cases. 18F-FDG PET has been extensively studied in these patients with a sensitivity ranging from 68 to 79 % [32]. The principal constraint in using FDG-PET for evaluation of secondary metastasis is the frequent hypometabolic nature of cerebral metastases, and in addition, metastatic lesions are often small (<1 cm in size), and because metastases most often occur at the interface between grey and white matter, identification of lesions can be challenging.
FLT could be used in a limited number of cases suspected of rapidly progressing proliferating brain tumor. C-11 methionine is another radionuclide that is being extensively evaluated for its application in the detection of brain tumor. Unlike FDG, the uptake of 11C-methionine in the human brain is almost negligible. Since the proliferation rate of many brain tumors is relatively low, radiolabeled amino acid analogues are more sensitive than FLT [10]. Currently, the production yield of 11C-labeled radioligand is far below its requirement, thus its practical application is faced with difficulty. F-18 fluoroethyltyrosine (F-18 FET) is a promising alternative fluorinated amino acid analogue whose preliminary studies in brain tumor evaluation appear promising [33].
Lung Carcinoma
There have been numerous studies examining the accuracy of 18F-FDG PET in evaluating solitary pulmonary nodules [34–36]. Analyzing the published data has shown a high sensitivity of 96 % and accuracy with 94 % in determining this malignancy [16, 23, 37]. staging in non-small cell lung carcinoma (NSCLC) is very crucial in treatment planning. Studies that have evaluated the role of 18F-FDG PET for staging have reported sensitivity of 82–100 % and specificity of 73–100 % [25, 30, 38–41]. In all series, 18F-FDG PET has been shown to outperform CT in staging lymph node spread in up to 24 % of patients [39]. False-negative results often arise where small lesions are present (<0.6 cm), due to the resolution limitations of PET scanners, respiratory motion over the acquisition period or with certain types of lung cancer such as bronchoalveolar and carcinoid. Figure 14.1a, b shows PET/CT images with DOTATOC tracer from a patient with recurrent lymph node metastases with primary lung cancer. Figure 14.2a, b shows PET/CT image from a patient with pulmonary carcinoid tumor in left lower lobe with metastases to liver. DOTATOC (DOTA(0)-Phe(1)-Tyr(3)-octreotide) is a highly specific PET tracer to somatostatin receptor (SSR2) of neuroendocrine tumor [42, 43].
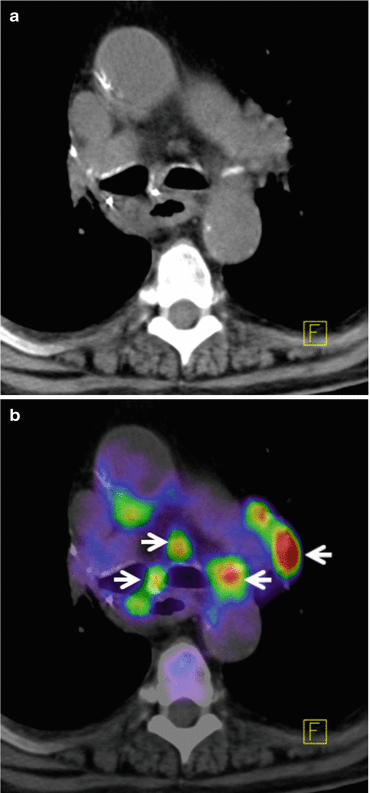
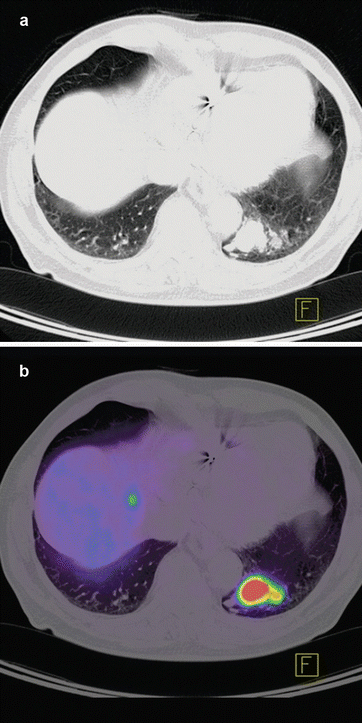

Fig. 14.1
(a) Axial slice from CT thorax level of the mediastinum presenting a patient with recurrent lymph node metastases from primary lung cancer. (b) Co-registered PET/CT images with high FDG tracer uptake indicating lymph node metastases shown with arrows

Fig. 14.2
CT (a) and PET-CT (b) axial slice of chest from a patient with pulmonary carcinoid tumor in the left lower lobe. The neuroendocrine differentiation of the tumor leads to an intense somatostatin receptor (SSR2) overexpression on which the somatostatin analogue 68Ga-DOTATOC tracer can bind. Physiologically SSR2 is expressed only in pituitary, thyroid, adrenals, and excretory organs; any other representation is considered pathological
Colorectal Carcinomas
Primary colorectal cancers occasionally present as an incidental finding on 18F-FDG PET, and 18F-FDG uptake has been reported in adenomatous polyps, a precursor for colon cancer [44]. However, the presence of physiological gut uptake of FDG combined with false-positive uptake in inflammatory disease along with low sensitivity to lesions less than 1 cm precludes a significant role for FDG-PET in primary diagnosis or screening [45]. The role of PET in primary colon cancer remains limited and should be reserved for clinical situations where resection of metastatic disease requires accurate staging of distant spread. PET is an excellent tool in detecting secondary metastasis to liver or extrahepatic abdominal metastases. In published series, the accuracy of 18 F-FDG PET in identifying metastatic colorectal carcinoma in the liver has ranged from 90 to 98 % [23] and in extrahepatic diseases, it ranges from 92 to 93 %. In patients with elevated serum CEA markers, occult disease (often extrahepatic recurrence) has been identified accurately with 18F-FDG PET [46].
In advanced rectal cancer, where 18F-FDG PET has been shown to have a significant impact on management in up to one third of patients planned for preoperative adjuvant treatment (chemoradiation), indicating the potential role of 18F-FDG PET in this clinical setting [47].
Lymphoma
The sensitivity and specificity of 18F-FDG PET in detecting the sites of lymphoma has been reported as 86 %–90 % and 93 %–96 % respectively and is considerably superior to CT scans. 18F-FDG PET aids in staging Hodgkin’s and non-Hodgkin’s lymphoma (NHL) with high acuity. [4, 16, 23]. 18F-FDG PET has been also shown to change the management in up to 40 % of patients undergoing staging at initial diagnosis [48–51]. In comparison to 67Ga scans, 18F-FDG PET has been shown to have a greater sensitivity for disease detection (particularly spleen) and in view of the potential advantage of a same-day procedure has supplanted 67Ga scans in many oncology centers [52]. 18F-FDG PET is now routinely performed as part of the assessment of treatment response for NHL; it has also shown to be superior to conventional imaging and to be a strong prognostic indicator of response and progression-free survival [53, 54]. 18F-FDG PET therefore has a major role in both the initial staging and restaging/therapy response assessment of patients with lymphoma. Figure 14.3 shows PET images before, during, and after chemotherapy from patient with Hodgkin’s lymphoma.
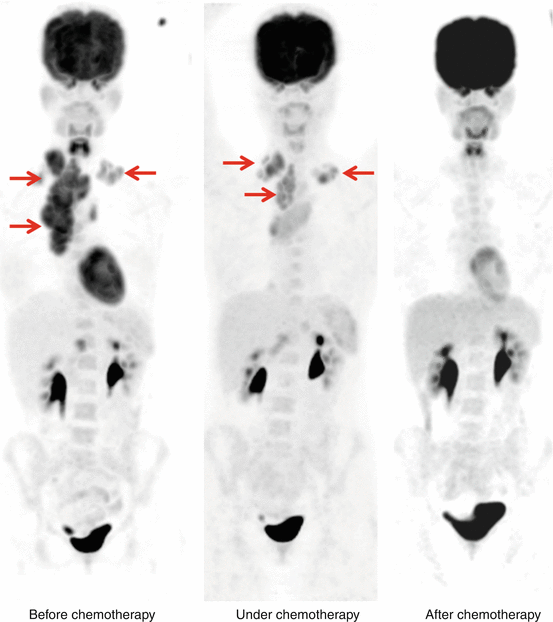

Fig. 14.3
Patient with Hodgkin’s lymphoma before (left panel), under (middle panel), and after several cycles of chemotherapy (right panel) showing high FDG uptake of in the cervical and mediastinal region (arrows) which decline after several cycle of chemotherapy—metabolic component (FDG-PET) is the leading imaging modality in these tumor entities for tumor response assessment
Melanoma
Malignant melanoma can spread widely and unpredictably throughout the body, and median survival after the appearance of distant metastases is approximately 6 months [55] which makes it very critical to be detected at an early phase. The accuracy of 18F-FDG PET in detecting metastatic melanoma has been reported to range from 81 to 100 %, and in one series of 100 patients demonstrated a sensitivity of 93 % [27]. 18F-FDG PET has been shown to be particularly sensitive in detecting subcutaneous and visceral metastases (Fig. 14.4). In published studies and meta-analyses of the literature, 18F-FDG PET has been demonstrated to detect disease up to 6 months earlier than conventional techniques and alter management in 22–32 % of patients, principally by altering plans for surgical resection of metastatic disease [27, 56, 57]. The role of 18F-FDG PET in melanoma is therefore principally in the evaluation of extent of metastatic disease, the accurate assessment of which can alter patient management particularly where surgery is planned.
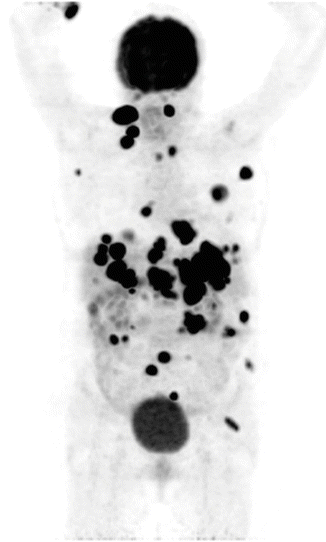

Fig. 14.4
Maximum intensity projection in coronal plane of body presenting FDG PET uptake in a patient with metastasized malignant melanoma
Head and Neck Tumors
The presence of lymph node spread of head and neck tumors is associated with substantially worse prognosis which needs to be addressed in early phases. In patients with head and neck tumors studied prior to initial surgery, the sensitivity and specificity of 18F-FDG PET in detecting nodal metastases has been reported ranging from 71 to 91 % and 88 to 100 %, respectively [23, 58–60]. In patients studied after initial treatment of metastatic nodal disease with radiotherapy, 18F-FDG PET is often accurate only after a 3-month period [35, 61–63]. In both patient groups, the accuracy of PET has the potential to direct surgeons to otherwise unexpected sites of metastatic disease, as well as in avoiding surgery at areas where the scan is negative. 18F-FDG PET has also been shown to be a prognostic factor for radiotherapy response [64].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree