1. Define the terms radioactivity, radionuclide, and radiopharmaceutical.
2. Explain the structural difference between radioisotopes and stable isotopes.
3. Define the terms activity, radioactive decay, and physical half-life.
4. Name five modes of radioactive decay. For each mode, name the nuclear deficiency of the parent, the common emissions, and an example radionuclide.
5. Describe the function of a radionuclide reactor. State the most common nuclear deficiency of reactor-produced radionuclides and their decay mode. Provide examples of two reactor-produced radionuclides.
6. Describe the function of a cyclotron. State the most common nuclear deficiency of cyclotron-produced radionuclides and their decay mode. Provide examples of two cyclotron-produced radionuclides.
7. Describe the function of a radionuclide generator. Define the terms transient equilibrium and secular equilibrium. Provide examples of two generator-produced radionuclides.
8. Name five mechanisms of radiopharmaceutical localization and provide an example radiopharmaceutical for each.
9. Describe the effects of half-life and gamma photon energy on patient radiation dose.
10. Define the terms deterministic effect and stochastic effect.
11. Describe a scenario leading to a medical event, and the reporting requirements in a Non-Agreement State.
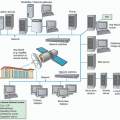
for increased diagnostic accuracy of nuclear imaging. SPECT/CT, PET/CT, and PET/MRI are powerful hybrid imaging tools.
as an activity of 1 disintegration per second (dps). The curie (Ci), named after radioactivity pioneers Pierre and Marie Curie, is the traditional unit of activity equal to 3.70 × 1010 dps. Thus,
99mTc has a physical half-life of 6.02 hours. Approximately how much of a sample of 99mTc remains after 24 hours?
24 hours represents almost four half-lives.
A = A0/2(4) = A0/16.
Approximately 1/16th of the original activity remains after 24 hours.
If 20 mCi of 99mTc sestamibi is administered to a patient, how much activity remains after 24 hours, assuming no physiologic clearance?
20 mCi/16 = 1.26 mCi
As a rule of thumb, a radioactive sample will decay to approximately 1/1000th its original activity after 10 half-lives (210 = 1024). How long will it take a sample of 99mTc to decay to 1/1000th of its original activity?
For 99mTc, 10 half-lives is approximately 60 hours, or 2½ days.
At 8 PM on Friday evening, a 100 mCi vial of 99mTc pertechnetate is spilled on a carpeted surface in the nuclear medicine department, completely soaks in, and cannot be removed. How much activity remains when the first patient shows up at 8 AM Monday morning, 2½ days later? (Figure 8.3).
100 mCi/1000 = 0.1 mCi = 100 µCi
Table 8.1 PHYSICAL HALF-LIFE (Tp½) AND DECAY CONSTANT (λ) FOR RADIONUCLIDES USED IN NUCLEAR MEDICINE | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 8.2 RADIOACTIVE DECAY MODES FOR UNSTABLE NUCLEI CONTAINING PROTONS (Z), NEUTRONS (N), AND MASS NUMBER (A) | ||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||
Below this threshold, positron emission does not take place, but the neutron-deficient isotope may undergo electron capture, discussed later. Above the threshold, electron capture may compete with positron emission. This threshold is also reflected in the minimum mass difference between the parent and daughter atoms required for positron emission. Like the antineutrino seen in β– decay, the neutrino does not significantly interact with the patient or the clinical nuclear imaging system (Figure 8.10).
 FIG. 8.7 • Principal decay scheme of Mo-99. The many possible radiation decay products are listed on the accompanying decay data table. |
and does not involve the gain or loss of particles as in other decay modes discussed (Figure 8.12).
Table 8.3 FDA-APPROVED PET RADIOPHARMACEUTICALS | ||
|---|---|---|
|
 FIG. 8.11 • Electron capture. A neutron-deficient radionuclide incorporates an inner shell electron into the nucleus with emission of a neutrino (ν) and a gamma ray. The vacancy may be filled by an outer shell electron and will result in characteristic X-rays or Auger electrons. The daughter nuclide has a decreased atomic number and an unchanged atomic mass number.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|