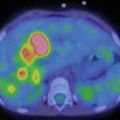
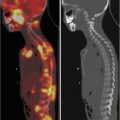
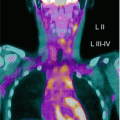
Fig. 6.1
Male, 9 years, anomalous origin of the left coronary artery. MIBI myocardial perfusion scintigraphy. Reversible hypoperfusion in the anterior wall of the left ventricle
6.2.6 Transposition of the Great Arteries
Myocardial perfusion imaging has been frequently employed in children [27, 28] after surgical intervention involving mobilization and/or reimplantation of coronary arteries, as in the arterial switch operation (ASO) for the transposition of the great arteries (TGA), where the coronary arteries are reimplanted at the time of surgery and may be prone to kinking, abnormal vasodilation, or failure to grow at the anastomosis level after reimplantation. Severe hypoperfusion, fixed or reversible, is usually associated with perioperative complications and brings a poorer prognosis. Perfusion defects can be detected frequently during the follow-up of patients treated with ASO, most commonly in the apical, lateral free wall of the left ventricle [28–30]. In most cases their size is small, and they have no influence on the ventricular performance. Their pathophysiologic significance (i.e., microcirculation disturbances or reduced endothelial function) and their prognostic value have not been yet established. Myocardial perfusion scintigraphy has been used also in the follow-up of TGA patients treated with the Mustard-Senning procedure (atrial switch), where the morphological right ventricle is the systemic ventricle and therefore develops a progressive hypertrophy, which can be complicated by regional ischemia [31], as it has been described in univentricular correction (Fontan procedure) [32].
6.2.7 Metabolic Syndromes
Nuclear medicine techniques have been applied to Duchenne muscular dystrophy (DMD), which is associated with myocardial degeneration and fibrosis. Myocardial lesions are segmentally distributed and start the basal inferior and infero-lateral walls of the left ventricle, progressing to midinferior, apical, and anterior segments, where a severe transmural fibrosis and fatty infiltration has been observed, with increased glucose utilization on the FDG-PET study (perfusion metabolism mismatch) [33].
A recent study proved the utility of myocardial perfusion imaging in Williams syndrome, a multisystem disorder characterized by a deletion of the elastin gene, which leads to diffuse cardiovascular alterations, often involving the coronary arteries [34].
6.3 Lung
Pulmonary blood flow disturbances are a frequent finding in congenital heart disease. They have often heavy consequences on the blood saturation and on the development of the child, requiring prompt treatment and a prolonged follow-up. Furthermore, lung perfusion unbalance may be a complication of surgical manipulation of the pulmonary arteries during palliative and/or corrective interventions. Lung perfusion scintigraphy represents an effective and safe technique for the noninvasive study of pulmonary blood flow distribution and is a perfect complement to ultrasound techniques.
6.3.1 Radiopharmaceuticals
The distribution of pulmonary blood flow can be assessed by lung perfusion scintigraphy using technetium-labeled macro-aggregate of albumin (99mTc-MAA) or albumin microspheres [35, 36]. Standard adult radioactivity dose must be reduced according to radiation protection regulation, preferably referring to a validated dose reduction algorithm [i.e., EANM dose calculator or similar]. A similar reduction in the number of injected particles (Table 6.1) is required, to avoid a significant increase in pulmonary vascular resistance, even in infants with severe pulmonary hypertension. The method has proved to be safe even in patients with known significant right-to-left shunts, where a further prudential reduction in the number of injected aggregates may be advisable, to limit to a minimum dissemination in the systemic circulation. The total amount of injected particles should not be less than 10,000–20,000, to avoid a significant deterioration of image quality [37].
Table 6.1
Suggested numbers of injected particles for lung perfusion scintigraphy in children
Body weight | <5 kg | 6–15 kg | 16–20 kg | 21–35 kg | >35 kg |
Particles number | 10,000–50,000 | 50,000–100,000 | 100,000–200,000 | 200,000–300,000 | 300,000 |
6.3.2 Patient Preparation
No specific preparation is required for lung perfusion scintigraphy and sedation is not routinely indicated, at least for standard acquisition. It is highly recommended to defer the exam when a concomitant illness (i.e., bronchopulmonary infections) can interfere with tracer distribution. Even low-grade bronchoconstriction can result in significant redistribution of pulmonary blood flow [38], making difficult the interpretation of scintigraphic findings. Therefore, it is advisable to wait for the resolution of respiratory symptoms before performing lung perfusion scan. The tracer is administered via a peripheral vein, avoiding whenever possible injection in a venous line, which can lead to “hot spot” artifacts. The site of administration is not relevant when normal atrial mixing is present and both lungs are perfused through a common blood supply. When these conditions are not met, the injection site must be adapted to the physiology of the pulmonary blood flow, taking in account the actual functional anatomy of the single patient. This is the case of some complex malformations or after some types of surgical repair (i.e. staged Fontan procedure), when multiple injections are required [39] (vide infra).
6.3.3 Image Acquisition and Processing
Static images are acquired shortly after injection, in posterior and anterior views (200–500 kilocounts/frame, 256 × 256 matrix, acquisition zoom adapted to patient size), using ideally a parallel hole high-resolution collimator. Oblique views are obtained when necessary, to clarify dubious findings. Relative lung perfusion is usually computed using geometric mean counts from region of interest (ROI) on both lungs in anterior and posterior projections. However, calculations based on the single posterior projection have been shown to differ only slightly from values based on geometric mean [40]. Therefore it is possible to acquire only the posterior view, without losing significant clinical data, making easier the acquisition in infants and uncooperative children. Moreover data obtained from anterior projection can be sometimes misleading, due to the significant influence of heart position [41]. Extra-pulmonary tracer is usually negligible and background subtraction can be useful only in few selected patients, presenting significant right-to-left shunting and extremely hypoperfused lung. In this case background subtraction can correct for lung counts from extra-pulmonary tissue, but care must be taken to adopt the same approach in the follow-up studies. It is also possible to quantify a right-to-left shunt, comparing the lung counts with the total extra-pulmonary activity on a whole body image [42] or with the brain counts [43].
6.3.4 Typical Findings
The normal distribution for pulmonary blood flow usually in the 45–55 % range for the single lung (right + left = 100 %) and the ROI-based calculation is highly reproducible. The most frequent abnormality observed in congenital heart disease is a diffuse unilateral reduction of relative pulmonary blood flow, in most cases related to a stenosis of the left or (less frequently) right pulmonary artery. Nevertheless, the same pattern can occur by multiple peripheral stenoses of the main arterial branches or by diffuse vascular involvement affecting the whole lung; therefore, scintigraphic imaging represents only a step before the definitive diagnosis. Focal hypoperfusion, which is unusual in children with congenital heart disease, is linked more often to a single peripheral stenosis. In some cases an apical hypoperfusion is observed on the same side of Blalock-Taussig shunt, probably as a sequel of vascular distortion during the surgical procedure [44, 45]. This kind of abnormality can persist after removing the shunt, but it has usually small impact on the global distribution of the blood flow to the affected lung. A functioning Blalock-Taussig shunt can lead to a variable underestimation of the blood flow to the ipsilateral lung through a dilution effect of the systemic blood on the radiolabeled particles arriving via the pulmonary artery. The same dilution mechanism underlies the focal hypoactivity of lung segments perfused by persistent aorto-pulmonary connections, as is often observed in pulmonary atresia [46].
6.3.5 Clinical Indications
Lung perfusion scintigraphy has a limited role in the early diagnostic work-up of congenital heart disease, because diagnosis and treatment planning rely in most cases on morphological imaging (echocardiography, MRI, cardiac catheterization, etc.). The noninvasive evaluation of pulmonary blood flow distribution becomes critical after surgical palliation or correction, since an unbalance in lung perfusion may arise, without any clinical sign, even after successful uncomplicated one-stage correction of mild anomalies [47]. Echography can explore only the most proximal tract of pulmonary arteries; MRI or repeated angiographic studies are too invasive for simple follow-up purposes, but the combination of ultrasound and lung perfusion scintigraphy allows a prolonged follow-up with little biological and economical cost. Therefore lung perfusion scan is indicated in the follow-up of congenital heart disease whenever there is the need to evaluate the relative distribution of pulmonary blood flow [48]. The integration with ultrasound compensates for the inability of scintigraphy to detect a symmetric decrease in pulmonary blood flow, as in stenosis of the main trunk of the pulmonary artery or in bilateral balanced stenosis. On the other side, scintigraphy has the capability of look into the peripheral distribution of pulmonary blood flow without limitations related to anatomical anomalies. Typical indications for this approach are the tetralogy of Fallot (Fig. 6.2), the isolated stenosis of the pulmonary arteries, the monitoring after closure of a persistent ductus arteriosus or, as an emerging indication, the follow-up of anomalous pulmonary venous return. Even more relevant is the role of perfusion scanning in the follow-up of staged surgical repair, as in the correction following the principle of the Fontan circulation, where a single ventricle sustains both systemic and pulmonary circulation and the superior and inferior vena cava flow directly in the pulmonary arteries, through surgical anastomosis. In this situation it is mandatory to split the dose between arm and leg injection, to evaluate correctly the contribution of SVC and IVC to pulmonary blood flow. A single injection would give a falsely asymmetric distribution, with a preferential flow to the right lung from the SVC or a prevalent flow to the left lung after leg injection (Fig. 6.3) [48]. MRI imaging can give more reliable results when a complete Fontan circulation is present [49–52]. Stenosis of the pulmonary arteries may persist after each phase of the staged repair, or it may arise as a consequence of surgical manipulation. The effects of reduced blood flow on vascular and/or pulmonary development can be corrected and even reverted with prompt treatment (surgical reintervention or, more often, angioplasty and endovascular stenting). The combination of seriate echographic and scintigraphic studies allows to limit the use of cardiac catheterization, which can be employed only when angioplasty is required, leading to a significant reduction of radiation exposure.
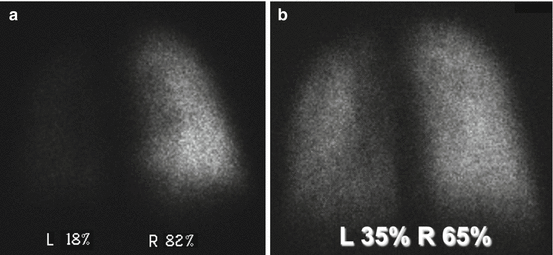
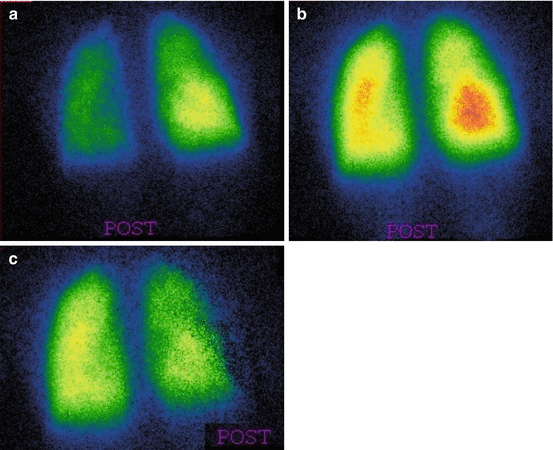

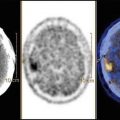
Fig. 6.2
(a) Male, 6 months. Repair of tetralogy of Fallot. MAA pulmonary perfusion scintigraphy showing marked hypoperfusion of the left lung. (b) MAA pulmonary perfusion scintigraphy 3 months after percutaneous balloon angioplasty of the left pulmonary artery. Marked improvement of the perfusion in the left lung

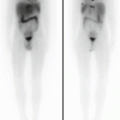
Fig. 6.3
(a) Female, 4 years. Fontan circulation (SVC and IVC anastomized to the pulmonary arteries). MAA pulmonary perfusion scintigraphy (split-dose) after injection in the upper limb: preferential distribution from SVC. (b) After injection in the lower limb, the lung perfusion is symmetric. (c) Subtraction image (summed injections – upper limb injection) showing the preferential contribution from the IVC to the left lung
References
1.
Robinson B, Goudie B, Remmert J, Gidding SS (2012) Usefulness of myocardial perfusion imaging with exercise testing in children. Pediatr Cardiol 33:1061–1068CrossRefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree