and Jurgen J. Fütterer2, 3
(1)
Department of Radiological Sciences, Oncology and Pathology, Sapienza University of Rome, Rome, Italy
(2)
Department of Radiology and Nuclear Medicine, Radboudumc, Nijmegen, The Netherlands
(3)
MIRA Institute for Biomedical Technology and Technical Medicine, University of Twente, Enschede, The Netherlands
Oncocytoma, Renal
Oncocytoma is the second most common benign renal neoplasm after angiomyolipoma. It accounts for 3–7 % of solid renal masses. They appear to originate from the cortical part of collecting tubules. These tumors are often detected incidentally in asymptomatic individuals; some are multiple, bilateral, or metachronous in a minority of cases. In rare cases, presenting as hundreds of nodules scattered throughout both kidneys.
CT: they are solid, well-marginated, homogeneous, and often large tumors. Cystic changes and calcifications are rare. They have no specific imaging features (such as MRI), in fact imaging cannot differentiate it from a malignant renal mass, unlike which, hemorrhage and necrosis are not common. Oncocytomas tend to contain a central stellate scar that mimics the necrosis seen in a renal cell carcinoma.
MRI: The MR appearance is variable and nonspecific. They are hypointense on T1-weighted MR images (70 % of the cases) but vary in signal intensity on T2-weighted images (67 % high signal intensity), often with a well-defined capsule. The central scar (when present) can be seen as a stellate area of low signal intensity on T1-weighted images and high signal intensity on T2-weighted images; Fig. 4a, b. Postcontrast MR imaging reveals, in a minority of oncocytomas, a “spoke-wheel” enhancement pattern. However, this is a nonspecific sign, because a similar vascular arrangement has been described with renal cell carcinoma.
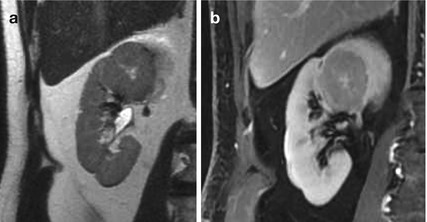
Fig. 4
(a, b) Oncocytoma in a 40-year-old woman. T2-weighted imaging and delayed contrast-enhanced T1-weighted images, showing a large, round-shaped, well-definied, mass with heterogeneous signal intensity and a central cleft. Oncocytoma was confirmed at histopathology analysis performed after nefrectomy
Orchidopexy
Orchidopexy is the most succesful surgical treatment of cryptorchidism that is characterized by the failure of descent of one or both testicles in the scrotum. Surgical intervention is aimed at positioning and fixing of one or both testicles. The undescended testes is associated with a greater incidence of neoplasia at this level since the retention leads to degenerative phenomena of the gonad with arrest of spermatogenesis. This procedure should be performed after the twelfth month of life, the period during which the testicle could still end his physiological descent into the scrotum.
Several complications may occur including testicular retraction, atrophy or infarction, hematoma formation, ilio-inguinal nerve injuring, postoperative torsion, and damage to the vas deference.
MR imaging can be used in case ultrasound is inconclusive. Anatomical T1- and T2-weighted imaging can be applied for scrotal and pelvic evaluation. The testis is high signal intensity on T2 and intermediate signal intensity on T1.
Orchitis
Most episodes of orchitis result from extensions of acute epididymitis. In isolated orchitis, a viral infection, such as mumps, should be suspected. Causative agent in adolescent and men younger than 35 years are usually considered as Chlamydia trachomatis and Neisseria gonorrhoeae. Less common causes of epididymitis and orchitis include Granulomatous conditions such as Tuberculosis, Sarcoidosis, and Brucellosis and chemical epididymitis in reflux of sterile urine, amiodarone therapy, or prostate brachytherapy. In children less than 2 years of age, a predisposing condition is usually identified, such as imperforated anus, ureteral ectopia to seminal vesicles, bladder exstrophy, and posterior urethral valves.
Complications of epididymo-orchitis are abscess formation, pyocele, infarct, gangrene, infertility, atrophy and chronic pain.
MRI: In epididymo-orchitis, the testis and epididymis have heterogeneous low signal intensities on T2-weighted images. The epididymis will be enlarged and hyperenhancing with contrast on T1-weighted studies. The testis may show inhomogeneous enhancement with hypointense bands.
Ovarian, Functional Cyst
Ovarian cysts are seen in all age groups, and many of them are functional cysts. Functional ovarian cysts include follicular cyst and corpus luteal cyst. Follicular cysts results from a failure of the follicle to rupture and corpus luteum cysts derive from hemorrhage in corpus luteum. Simple cysts have in general a size less than 3 mm. Unilocular cysts have a diameter less than 3 cm. Corpus luteal cysts may be larger and tend to be more symptomatic than follicular cysts. Functional cysts may spontaneously regress over time, usually within two menstrual cycles, and they should be monitored by follow-up US at 6–8 weeks.
MRI: Most ovarian cysts show a thin wall, with a very high signal intensity on T2-weighted images and intermediate to low signal intensity on T1-weighted images because of simple fluid content. Similar imaging characteristics may be seen in benign ovarian cystic tumors. The most helpful feature in distinguishing functional cysts from ovarian neoplasms is the presence of papillary projections and nodular septa in the latter. Larger simple cysts cannot be differentiated from unilocular cystadenoma, but if the cyst is more than 10 cm, a tumorous condition should be considered. The most helpful feature in distinguishing functional cysts from ovarian cystic tumors is the presence of papillary projections and nodular septa in the latter. Corpus luteal cysts have thicker walls than follicular cysts and avid enhancement, due to the thick luteinized cell layer that lines the interior of the cyst. Corpus luteum cysts do not demonstrate the profound T2 shortening that is seen with many endometriomas (T2-shading).
Ovarian Mature Cystic Teratoma
Teratoma, also known as dermoid cyst, is the most common ovarian neoplasm in woman under 45 years of age. “Mature” means benign, as opposed to the immature, malignant teratoma. Ovarian teratomas derive from germ cells and are classified into three main categories, among which the mature cystic teratoma account for 99 %. Less common types of mature teratomas are the monodermal teratomas, which include the struma ovarii and carcinoid tumors.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree