Basic protocol (without contrast media)
Advanced protocol (contrast media)
Localizer
TrueFISP transverse and coronal and sagittal, overlapping (2 mm slice thickness, effective)
T2 HASTE coronal and transverse (PACE)
T2 TSE (BLADE) FS transverse and coronal (PACE)
T1 VIBE transverse, no FS (3 averages)
T1 VIBE coronal, FS (3 averages)
Contrast media
T1 flash 3D (TWIST), 30 consecutive dynamic acquisition
Start of contrast media application parallel to image acquisition
T1 VIBE transverse and coronal, 3 averages, fat sat
TrueFISP coronal, dynamic (angulated between lung apex and diaphragmatic dome), temporal resolution 3 images/s
MRI scanners are available with different magnetic field strengths ranging from 0.2 to 3 Tesla for routine clinical use (even 7 Tesla are being used for human research applications). The lung consists of many air/tissue interfaces. These interfaces generate local field inhomogeneity leading to decreased signal. This so-called susceptibility artifact increases with increasing magnetic field strength. Therefore, a low field strength of 0.2 Tesla can be advantageous for lung parenchymal imaging; however, in the clinical setting 1.5 Tesla scanners are the most common and thus, most studies are done using these scanners.
2 Morphology
Morphological imaging is done using single shot or turbo spin echo (TSE) T2-weighted and gradient echo (GRE) T1-weighted images (Table 1) and can be accomplished within 25 min. Images should be acquired in at least two plane orientations as sometimes subtle pulsation or respiratory artifacts can hamper clear perception of the pathology. Especially, well-visualized pathologies are those with an increase in soft tissue or fluid relative to air, like inflammation. The inherent soft tissue contrast allows good differentiation between consolidations, effusions, and abscess formations without the use of contrast media. Even hilar and mediastinal lymph nodes are visualized well. Contrast media is only used in cases with suspected pulmonary perfusion abnormalities or in tumors.
Proton MRI is limited by the short transverse relaxation times of the lung parenchyma (T2 around 80 ms). Therefore, conventional T2 images are hampered by T2 blurring limiting assessment of fine-scale structures. Recently, 3D sequences facilitating ultrashort TE times were developed and combined with respiratory gating (Johnson et al. 2013). The spatial resolution can be as high as 1.25 mm isotropic. The initial results in healthy volunteers showed high quality visualization of the lung parenchyma. This approach yields great opportunities for future use of lung MRI, especially in the pediatric population.
2.1 Airways
Pathologies of the tracheobronchial tree in children are usually either due to a cartilaginous instability or an extrinsic compression. In children, the primary diagnostic tool for assessment of tracheomalacia is bronchoscopy or CT. However, if an external compression due to aberrant vasculature is suspected, MRI can be used, although the functional degree of stenosis is difficult to assess. MRI comes routinely into play after the trachea has reached a sufficient calibre to be visualized, in our experience at an age of 8 years. After surgery, MRI is capable of visualizing the tracheal lumen and assess for residual instability. Visualization in axial and coronal orientation should be accomplished (Fig. 1). T1-weighted sequences, like VIBE (volume-interpolated breath-hold examination) provide a spatial resolution of approximately 1.4 × 1.4 × 4 mm3 in a breath-hold of 18 s. T2-weighted TSE sequences have a spatial resolution of 0.9 × 0.7 × 3 mm3 and image acquisition is done during free respiration (Ley et al. 2010). After stent implantation, MRI is less useful as the metal leads to strong signal artifacts and loss of signal.
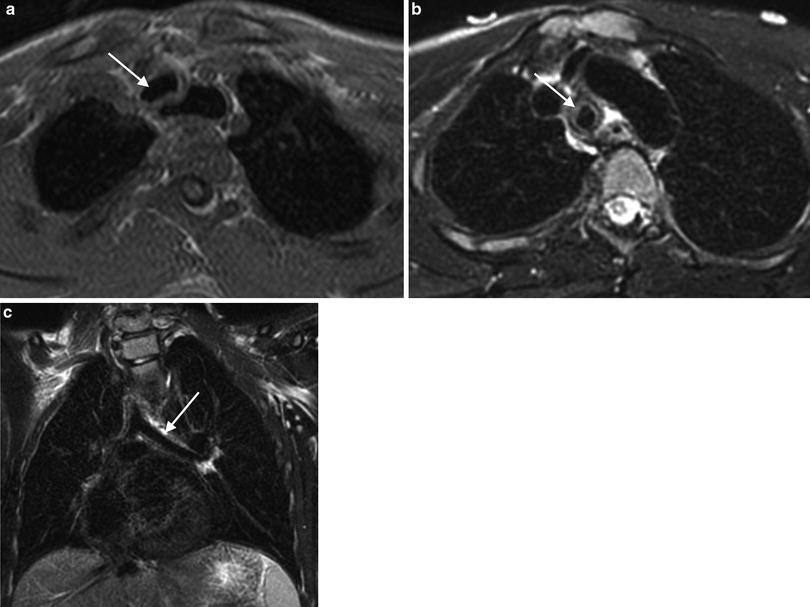

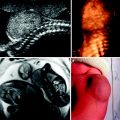
Fig. 1
Nine-year-old boy after tracheal stabilization surgery. a T1-weighted VIBE axial image. The arrow points to the trachea. b T2-weighted BLADE axial image. The trachea is designated by the arrow. c T2-weighted BLADE coronal image. The arrow points to the left mainstem bronchus. Even in free breathing, MRI is capable of depicting the trachea and proximal bronchi
For assessment of tracheal and diaphragmatic motion during free or forced respiration, dynamic, time-resolved techniques can be applied. Usually, a steady-state free precession sequence (spatial resolution 3 × 2 × 6 mm3, temporal resolution 3 images/s) is used. This allows easy visualization of diaphragmatic paresis after cardiothoracic surgery or lack of expiration in obstructive disease, as in patients with Swyer–James syndrome.
2.2 Pneumonia
As pointed out above, pathologies with an increase in soft tissue or fluid are easier to visualize on MRI than diseases with a deficiency of parenchymal structures (like alveolar simplification in patients with bronchopulmonary dysplasia). Therefore, the majority of publications on lung MRI are on the topic of pneumonia or cystic fibrosis.
MRI had a high agreement with CT (95 %) in the diagnosis of pneumonia in a study of adults (Eibel et al. 2006). In neutropenic patients, MRI showed the same findings as CT in 91 % of cases (Rieger et al. 2008). These positive reports about the capability of MRI are confirmed in our own experience (Fig. 2). However, although pediatric patients are radiosensitive subjects the use of MRI for pneumonia detection is rare. Usually, it is used in cases with a prolonged history or persistence despite antibiotic therapy (Fig. 3). In these cases, evaluation for a pulmonary abscess is needed and can be easily done by MRI. Also, characterization of pleural effusions is possible by MRI without contrast media, as well as planning of drainage placement.
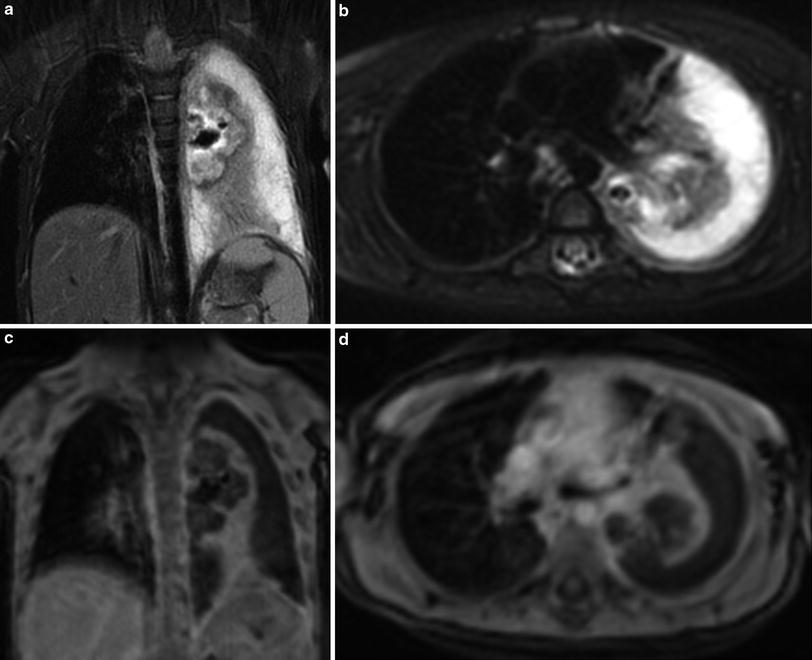
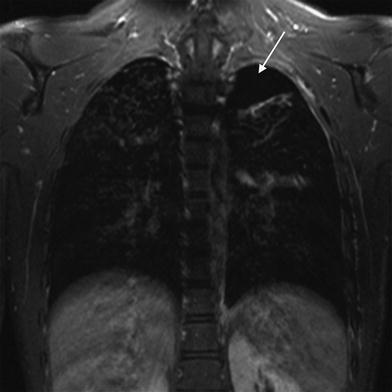

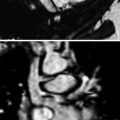
Fig. 2
Two-year-old girl with complicated pneumonia. There is a large left pleural effusion with septations. A large abscess is noted in the collapsed left lung. This abscess was not seen by ultrasound. T2-weighted BLADE coronal (a) and axial (b) images. Post-contrast T1-weighted VIBE coronal (c) and axial (d) images

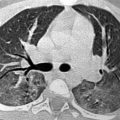
Fig. 3
Sixteen-year-old male patient with Langerhans cell histiocytosis and a history of recurrent pneumothoraces. The T2-weighted TSE image shows a left pneumothorax (arrow). In the right lung apex, typical cystic lung lesions are demonstrated
2.3 Cystic Fibrosis
Cystic fibrosis leads to chronic lung infection, airway obstruction, and progressive destruction of the lung parenchyma. These pulmonary pathologies are the main reason for hospitalization and reduced quality of life in cystic fibrosis patients. Frequent imaging studies, usually chest radiographs, are performed beginning in early childhood. This is a group of patients who could greatly benefit from MRI examinations of the lung as it allows morphological and functional assessment of disease activity without radiation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree