Fig. 1
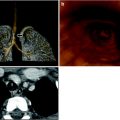
Normal variant of clavicles in a 15-year-old boy with fever of unknown origin. a Chest radiograph; close-up view of upper median aspect shows irregular sclerosis of right medial clavicular concavity, initially mistaken for osteomyelitis. b Axial CT scan through upper chest area at level of medial clavicular ends shows correlating irregular clavicular contours, especially on the right. No local soft tissue swelling. Subsequently, scintigraphy demonstrated osteomyelitis in the right distal femoral metaphysis
Isolated rib anomalies are common incidental findings, usually of no clinical importance, with an estimated frequency of about 2 % (Coury and Delaporte 1954). Such anomalies include partial aplasia or agenesis of ribs, bridging between two adjacent ribs by synostosis or pseudoarticulation, bifid ribs, and supernumerary ribs. Unilateral or bilateral cervical ribs may arise from the seventh cervical vertebra and, similar to anomalies involving the first and second ribs, can sometimes cause a thoracic outlet syndrome by compression of the brachial plexus or the subclavian artery (Schroeder et al. 2012) (Fig. 2). Intrathoracic rib is a rare anomaly that can be seen on chest radiographs (Kamaruddin et al. 1995). Eleven pairs of ribs occur in isolation or as manifestation of various syndromes like trisomy 18, Down syndrome, and cleidocranial dysplasia (Lachman 2006).
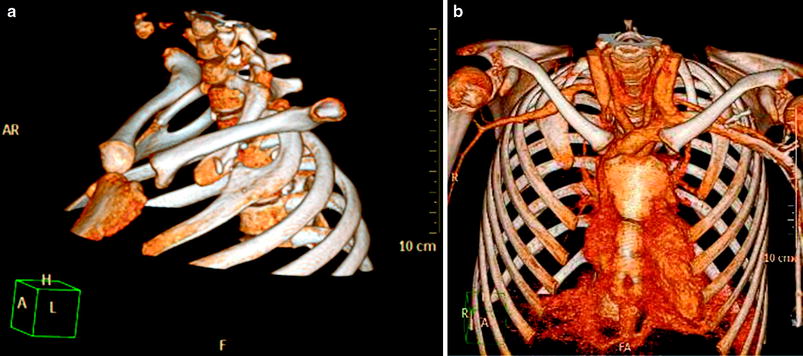

Fig. 2
Thoracic outlet obstruction in a 13-year-old girl presenting with Raynoud syndrome and decreased pulse pressure in the right arm. Left anterior oblique (a) and anterior (b) volume rendered views of contrast-enhanced CT demonstrate bilateral pseudoarticulation of the first and second ribs with compression and aneurysm formation of the right subclavian artery
Anatomic variations of the anterior chest wall are very common (Donnelly et al. 1999; Wong et al. 2004; Garcia-Peña and Barber 2010). Up to one-third of all children show asymmetry in the shape or size of the rib cartilage or in the position of the sternum. Usually, a palpable anterior chest wall bump is the cause for concern. The underlying anatomical cause may be a tilted sternum, or various anomalies of the rib cartilage such as a prominent anterior convexity, localized thickening, bifid cartilage, or a parachondral nodule. Even a mild degree of pectus excavatum or carinatum can produce a circumscribed protrusion that quite frequently prompts referrals for imaging studies. Of 27 children who underwent computed tomography (CT) or magnetic resonance imaging (MRI) for an asymptomatic, palpable chest wall bump, all had either benign lesions or normal variants of bone or cartilage formation in the anterior chest wall (Donnelly et al. 1997). Ultrasound (US) is an alternative method that can easily show the underlying anatomic variant and rule out a malignant chest wall mass for anxious parents and referring physicians (Fig. 3).
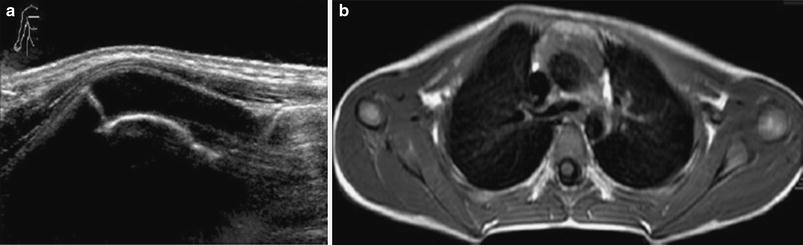

Fig. 3
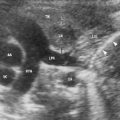
Rib deformity in a 6-year-old boy with “chest wall mass”. US scan (a) and axial T1-weighted MR image (b) through right upper thoracic area show redundancy of cartilaginous anterior rib portion (hockey stick shape)
2.2 Malformation and Deformity
Malformation of the chest wall may be a manifestation of a syndrome or skeletal dysplasia (Lachman 2006). Of particular interest are the neonatally lethal short rib-polydactyly syndromes, asphyxiating thoracic dystrophy (Jeune Syndrome), thanatophoric dysplasia, achondrogenesis, and other skeletal dysplasias in which maldevelopment of the thoracic cage produces a small and narrow chest due to short, and sometimes deformed ribs (Eich 2007; Glass et al. 2002). Respiratory distress at birth or even intrauterine death is directly related to the severity of the skeletal malformation.
A small thorax with thin ribs and small lungs can be a feature of neuromuscular disorders, particularly myasthenia gravis, myotonia, spinal muscular atrophy, and other myopathies. Thin ribs can be a feature of progeria and the trisomies 8, 13, and 18. Preterm infants show gracile ribs with posterior thinning. Thick ribs can be a manifestation of thalassemia (Cooley’s anemia), mucopolysaccharidosis, and other disorders. Inferior rib notching is due to abnormalities of the intercostal neurovascular bundle, such as arterial or venous collaterals (e.g., coarctation of the aorta, superior vena cava syndrome), and to neurogenic tumors (e.g., neurofibromatosis).
Rib aplasia or hypoplasia, when isolated, is of little clinical significance. Multiple hypoplastic ribs with or without additional spinal segmentation defects cause asymmetric deformity of the chest. Hypoplasia or aplasia of the lung may also cause an asymmetric thoracic cage.
Kyphoscoliosis can be idiopathic or congenital (due to vertebral segmentation defects), or it may be a complication of a neuromuscular disorder. The chest shows crowding of ribs on the concave side of the curvature and assessment of the heart and lung and may become difficult. It is not uncommon to find a smaller lung volume and atelectasis on the convex side. CT with three-dimensional (3D) reconstruction may be helpful for delineating vertebral anomalies and chest wall morphology (Bush and Kalen 1999). MRI may be indicated for screening the spinal cord for abnormalities prior to scoliosis surgery, if there is suspicion of spinal cord pathology clinically or in early onset idiopathic scoliosis (Koç et al. 2012).
Poland syndrome is characterized by unilateral partial or complete absence of the pectoralis muscles, hypoplasia of subcutaneous or breast tissues, hypoplasia or absence of ribs, and anomalies of the ipsilateral upper limb. On plain films the affected hemithorax appears hyperlucent. In the preoperative assessment of Poland syndrome, CT or MRI may help in defining the extent of the musculoskeletal and soft tissue anomalies and in showing the available muscles for reconstructive surgery (Wright et al. 1992; Cingel et al. 2013) (Fig. 4).
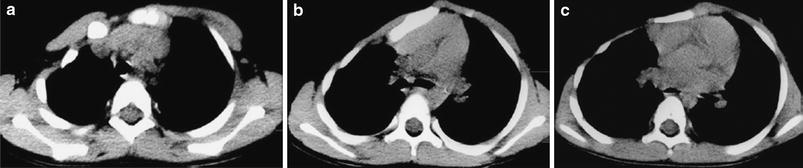

Fig. 4
Poland syndrome in a 4-year-old boy. Unenhanced axial CT scans at three different levels (a–c) show hypoplasia of major and minor right pectoralis muscles and right hemithorax with asymmetry of rib cage and sternum
Cleidocranial dysplasia, an autosomal dominant inherited syndrome, is characterized by hypoplasia or absence of one or both clavicles resulting in hypermobile, drooping shoulders. Other features of the chest wall consist of small scapulae, deficient sternal ossification, posterior wedging of thoracic vertebrae, scoliosis, kyphosis, and short ribs with prominent downward slope. Leading features of cleidocranial dysplasia are brachycephaly, wide sutures, persistence of the anterior fontanelle, abnormal dentition, absent or delayed ossification of pubic bones, and wide pubic symphysis (Lachman 2006).
Congenital pseudarthrosis of the clavicle is an isolated anomaly of the clavicle. This rare anomaly presents in infancy with a painless palpable mass. The clavicle shows a smoothly marginated defect in the middle third, virtually always on the right side (Fig. 5). There is no history of a prior trauma. Pseudarthrosis may be caused by the failure of two primary ossification centers to fuse (Cadilhac et al. 2000).
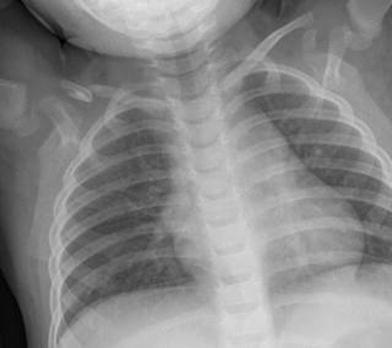

Fig. 5
Congenital pseudoarthrosis of the right clavicle in a 2-year-old girl without history of previous trauma
In Sprengel deformity the scapula fails to descend from its cervical origin and becomes fixed to the cervical spine by a fibrous band or an omovertebral bone. The scapula is high in position medially and rotated. Additional anomalies of ribs or vertebrae are frequently present (Klippel-Feil syndrome). CT with 3D reconstruction or MRI can be helpful in delineating the deformity and in planning corrective surgery (Cho et al. 2000; Dilli et al. 2011).
Pectus excavatum, also known as “funnel chest”, is the most common chest wall deformity. It is usually an isolated lesion that occurs sporadically or it may be inherited with an autosomal dominant trait. It can be associated with Turner syndrome, osteogenesis imperfecta, muscular dystrophy, or with connective tissue disorders like Marfan and Ehlers-Danlos syndromes. The lower portion of the sternum shows an inward curvature with a relative protrusion of the attached costal cartilages on each side. The sternum is usually rotated to the right. The characteristic radiographic findings are easily recognized (Fig. 6). On the antero-posterior view of the chest radiograph, the anterior rib ends have a steep downward course, while the posterior ribs are more horizontally oriented. The heart is shifted to the left and rotated. The right parasternal soft tissues produce a paracardial density and partially obscure the right heart border by a silhouetting effect. This should not be mistaken for middle lobe disease. On the lateral view, the chest is narrow and the degree of the sternal depression is easily seen. Cross-sectional imaging is useful to determine and quantify the severity of the deformity, to assess the degree of cardiac shift or compression, to identify associated tracheobronchial compression, and to assess the results of surgery (Pretorius et al. 1998; Calloway et al. 2011). Thoracic dimensions in patients with pectus excavatum are quantified by the pectus- or Haller index, which is the ratio of the internal transverse diameter of the chest to the narrowest antero-posterior diameter that commonly is calculated from a single axial scan or a limited CT study (Haller et al. 1987; Chuang and Wan 1995). The same measurements can be obtained without ionizing radiation from an axial MR image (Marcovici et al. 2011; Birkemeier et al. 2011; Lo Piccolo et al. 2012) (Fig. 7). Besides morphologic assessment, MRI also allows dynamic assessment of the chest wall and diaphragm (Raichura et al. 2001; Herrmann et al. 2006).
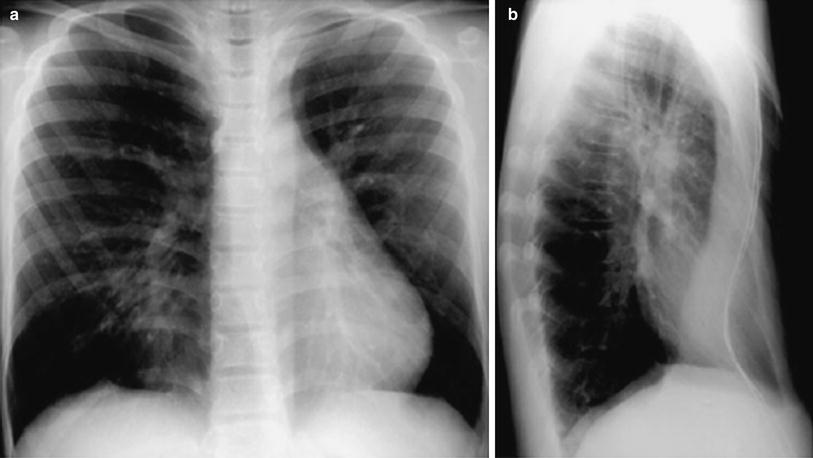
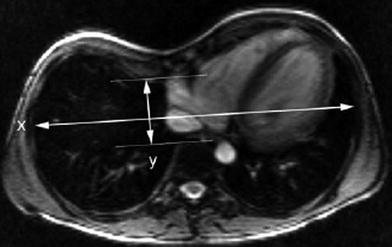

Fig. 6
Pectus excavatum. Postero-anterior (a) and lateral (b) chest radiographs in a 14-year-old boy show steep course of elongated anterior ribs, cylindric shape of chest and displacement of heart to the left due to reduced mid-sagittal diameter of chest. The outline of the sternum is enhanced with barium

Fig. 7
Axial steady-state free precession MR image of lower chest region in 11-year-old boy with pectus excavatum shows depression of the sternum and cartilagenous portion of a right rib, and leftward displacement but no compression of the heart. The Haller index is 5.1 and calculated as the maximal internal transverse diameter between rib cortices (x) divided by the minimal anterior-posterior diameter between the deepest point of the chest wall and the anterior cortex of the vertebra (y)
Patients requiring surgical correction of pectus excavatum usually have a Haller index greater than 3.2, whereas in normal children the Haller index values range from 1.9 to 2.7 due to age-related and sex-related differences in chest wall configuration. The Haller index in normal children under 2 years of age is significantly lower than in older children, and girls between the ages of 0–6 and 12–18 years tend to have higher Haller index values than boys of the same age (Daunt et al. 2004). In rare cases, respiratory or cardiac symptoms may be present, but most patients with pectus excavatum are asymptomatic and surgical correction is performed for cosmetic reasons. Restrictive lung volumes may not alter following operation, but cardiorespiratory function can increase due to higher cardiac output (Haller and Loughlin 2000).
Pectus carinatum or “pigeon breast” is a congenital or acquired deformity that develops with growth and is frequently seen with congenital heart disease (voussure cardiaque) (Shamberger et al. 1988) (Fig. 8). Other causes include long-standing obstructive lung disease, Marfan syndrome, Ehlers-Danlos syndrome, Noonan syndrome, Morquio syndrome, or prune belly syndrome, among others. The deformity seems to be caused by growth disturbance of both the sternum and costal cartilages with premature sternal fusion (Haje et al. 1999). The short sternum and costal cartilages protrude anteriorly with flattening of the chest laterally (Fig. 9). Most patients with a congenital pectus carinatum are asymptomatic. Surgery can correct the deformity.
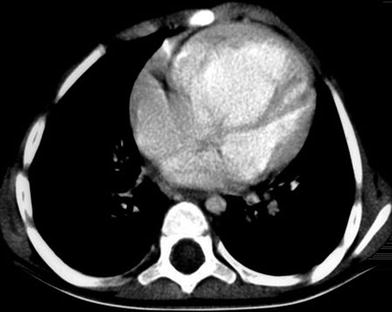
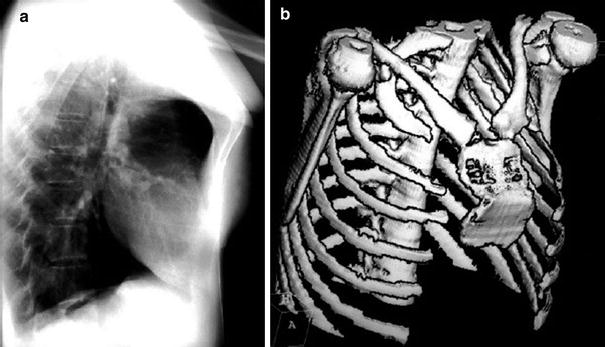

Fig. 8
Cor pulmonale (“voussure cardiaque”) in a 2.5- year-old boy. Axial contrast-enhanced CT scan through lower thoracic region shows (chronic) cardiac enlargement leading to increased sagittal diameter with additional leftsided protuberance of the chest. The child had primary pulmonary hypertension

Fig. 9
Pectus carinatum in a 17-year-old girl. a Lateral chest radiograph shows protrusion of upper and mid portions of the sternum. b Three-dimensional surface rendered CT reconstruction demonstrates correlating severe sternum deformity
Herniation of thoracic contents occurs when there is a defect in bony or soft tissue structures of the chest wall. Cleft sternum is a rare congenital lesion caused by partial or complete failure of sternal fusion at an early stage of embryonic development. Depending on the location and degree of the defect, herniation of thymus or the heart can be present (ectopia cordis) (Morales et al. 2000). Association with craniofacial hemangiomas and omphalocele are common associated anomalies (Fokin 2000). Lung hernia is a protrusion of pulmonary tissue through a defect of the chest wall. It may be cervical and intercostal in location. The more frequent intercostal hernia is mostly acquired following chest tube placement, surgery, trauma, chest wall neoplasm, or infection, but it can also be due to a congenital chest wall defect (Fig. 10). Cervical or apical hernia is associated with chronic obstructive lung disease in adults. In infants and children it arises spontaneously as a result of a congenital defect in the costovertebral fascia. The main symptom is an intermittant bulging in the supraclavicular or intercostal area that appears with crying, coughing, or straining. Chest radiographs or CT performed during inspiration may fail to show the lung herniation. Fluoroscopy during crying, coughing, or Valsalva maneuver is valuable in diagnosing lung hernias (Thompson 1976).
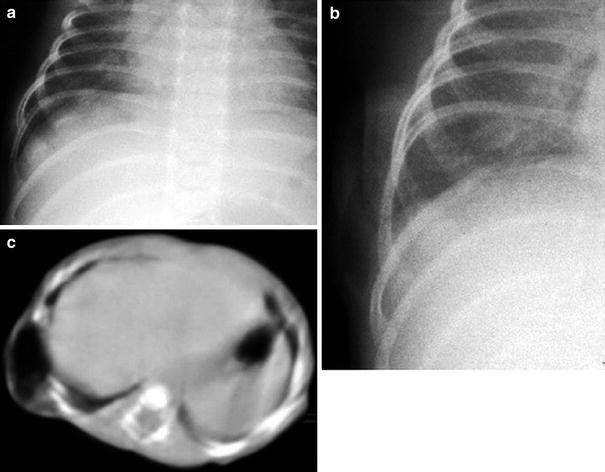

Fig. 10
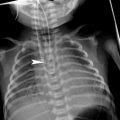
Lung herniation in a 4-week-old girl. a Chest radiograph at rest shows increased space between right ribs 9 and 10 and no lung prolapse. b On repeated chest radiograph while crying, lung herniates between the two ribs. c Axial CT while crying shows the herniated lung
3 Infection
Primary infection of the chest wall is relatively rare in children, but it is potentially fatal since secondary sepsis or spread to the pleural spaces, the mediastinum (Fig. 11), or pericardium can occur. Chest wall infection originates from hematogenous spread of organisms with sepsis or bacteremia, or from direct extension from a wound after injury or surgery (sternotomy) to the chest. Staphylococcus aureus is the most prevalent organism in chest wall infections of patients from Europe or North America (Sharif et al. 1990). Mycobacterium tuberculosis (Fig. 12) may be more prevalent in other areas of the world (Wong et al. 2004). Other microorganisms (Actinomyces, Blastomyces, Nocardia, and Aspergillus species) and cat-scratch disease can occasionally cause chest wall infections (Golladay et al. 1985; Lew and Waldvogel 1997). Chest wall infections are especially common in immunocompromised patients (Thomas et al. 2003).
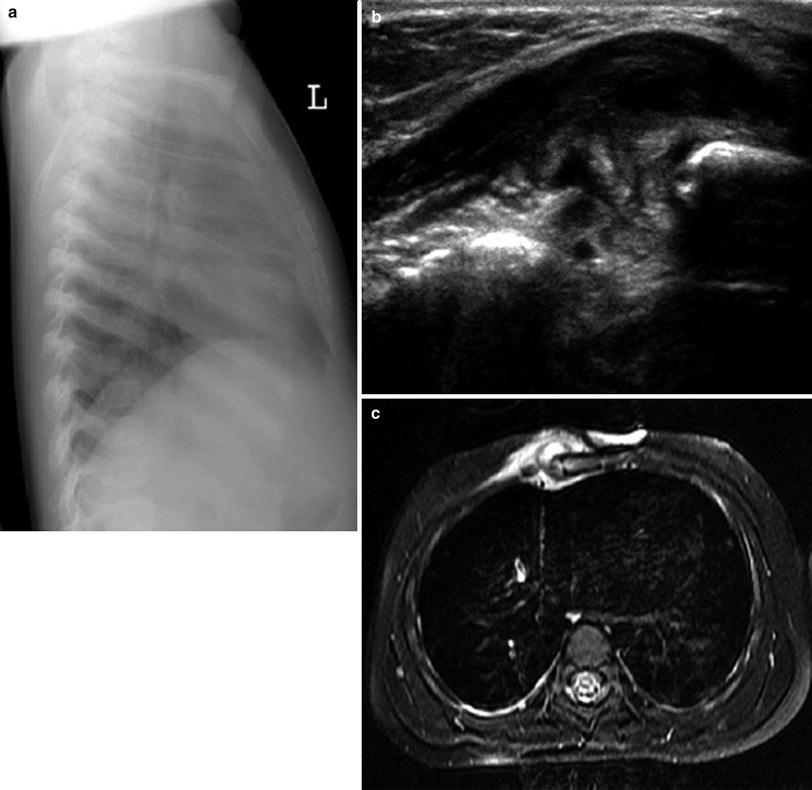
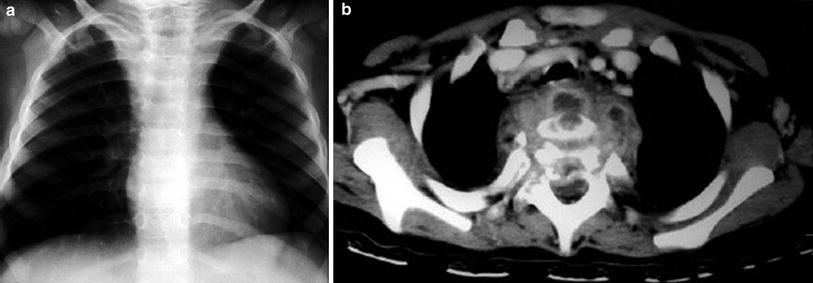

Fig. 11
Osteomyelitis of the sternum of a 1.5-year-old boy. a Lateral chest radiograph shows soft tissue swelling around the lower part of the sternum. b Transverse US image confirms the swelling and hypoechoic fluid collections below the pectoralis muscle and in subperiostal location at the sternum. c Axial fat-saturated T2-weighted MR image shows swollen soft tissue with mildly increased signal around the sternum, a small soft tissue abscess with high signal, and increased bone marrow signal in the sternum, consistent with osteomyelitis

Fig. 12
TBC abscess with vertebral osteomyelitis in a 2.5-year-old girl. a Chest radiograph shows unusual prominent shape of upper mediastinal region. b Contrast-enhanced axial CT scan through upper chest area shows complex paraspinal inflammatory mass with multiple abscesses involving vertebra, spinal arch, and spinal canal
Clinical symptoms include fever, pain, and focal signs of inflammation such as edema, erythema, hyperthermia, and occasionally fistulous tracts. Infection can involve the soft tissues and/or bone and cause abscess formation, cellulitis and/or granulation tissue formation. Depending on the structure preferentially affected it is called pyomyositis when muscles are involved, (necrotizing) fasciitis when only subcutaneous fat and fascia are affected, osteomyelitis when there is bone involvement, and pyogenic arthritis where there is joint involvement.
Clinical recognition of a chest wall infection can be difficult, particularly when it is located in the intermediate or deep layers of the chest wall (Garcia-Peña and Barber 2010). The underlying process is often underestimated by physical examination alone. A suspected (or unsuspected) infection of the chest wall is usually first imaged with chest radiographs, which may show a mass lesion within the chest wall or the extrapleural space. Additional signs that may also be present include rib destruction and/or sclerosis, pulmonary infiltrate, pleural effusion, and calcifications, air, or gas within the soft tissues. US, CT, and MRI help to confirm the presence, location, and extent of the single or multiple infectious foci (Figs. 11, 12 and 13). Positron emission tomography (PET) is a very sensitive tool for detecting clinically silent foci of infection in immunocompromised patients. US, CT, and MRI show fluid collections and rib destruction, and can guide percutaneous aspiration or drainage. US is usually sufficient for diagnosing small, superficial and well-delineated lesions, while CT or MRI are the techniques of choice for imaging large, complex, and deep-seated lesions for which surgery is considered. Intraspinal epidural extension may only be visible with CT or MRI (Fig. 12).
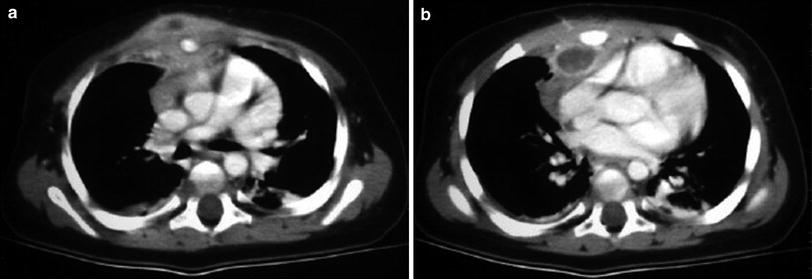

Fig. 13
a, b Osteomyelitis of the sternum due to Salmonella sp. following gastroenteritis in a 1-year-old boy. Clinical examination showed presternal swelling. Axial contrast-enhanced CT images show subperiostal fluid collections of the sternum as well as pre- and retrosternal abscesses
An abscess appears as a sonolucent area on US with increased through transmission and absence of blood flow centrally. Echogenic swirling material within the abscess may occasionally be seen. Contrast-enhanced CT shows an iso- or hypodense, nonenhancing center, and an enhancing rim (Figs. 11, 12, and 13) (Faro et al. 1993), similar to that seen on T1-weighted MRI sequences. T2-weighted and short tau inversion recovery (STIR) sequences show a high signal intensity collection. A moderate increase in signal intensity on T2-weighted sequences may be present in mycotic infection (Sharif et al. 1990). MRI is very sensitive for detecting osteomyelitis and is often more accurate than bone scans for differentiating between soft tissue inflammation and acute osteomyelitis. Chronic osteomyelitis is recognized on radiographs and CT as an area of destruction and reparative sclerosis within and around the affected part of bone. Inflammation of the surrounding soft tissues (cellulitis) appears as thickening in all modalities. In addition to this, US shows increased soft tissue echogenicity and vascularization, while CT and MRI show contrast enhancement. Soft tissue inflammation is best delineated by MRI with fat-suppressed T2-weighted or STIR sequences, or T1-weighted sequences following administration of contrast material, which provide better differentiation from the unaffected subcutaneous fat. In our experience, septic arthritis of the sternoclavicular joint is usually associated with osteomyelitis of the adjacent clavicle or sternum. CT and MRI show the joint effusion and osteolytic changes of the affected bone more readily than US and they can confirm or exclude posterior extension of the process into the mediastinum (Figs. 11 and 13).
Tuberculous spondylitis is relatively rare in developed countries, but it is the commonest vertebral infection in other parts of the world. The spinal infection mostly stems from primary pulmonary tuberculosis. One or several segments of the spine may be involved, particularly in the thoracic and lumbar region. Usually, the infection is limited to the body of the vertebra, which may become destroyed along with the contiguous intervertebral disk and an adjacent or distant vertebra. Paraspinal abscesses, usually bilateral, are the rule. Calcification within a paraspinal abscess can occur in long-standing cases (Kuhn 2003). Vertebral collapse can lead to kyphosis and/or scoliosis and even to cord compression. The radiographic changes of tuberculous spondylitis are nonspecific, but an indolent presentation is suggestive of tuberculosis. CT and MRI can show the epidural extension of the process and delineate the topography of an abscess (Fig. 12).
Friedrich’s disease is a disorder of unknown origin thought to be an aseptic necrosis with clinical and radiologic features that can mimic infection at the sternoclavicular joint (Levy et al. 1981). The lesion is usually unilateral but may be bilateral. Tender swelling at the sternoclavicular region is the typical presenting symptom. The erythrocyte sedimentation rate may be elevated. Radiographs show destruction and repair at the medial end of a clavicle (Fig. 14). Histology discloses necrosis of the clavicular epiphyseal region without evidence of infection. Aspiration cultures are negative. The symptoms usually subside spontaneously without treatment over several months. Radiological features improve very slowly. Our experience has shown that it may take up to 18 months for the clavicles to become radiologically normal.
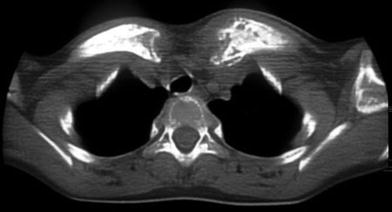

Fig. 14
Friedrich’s disease in a 9-year-old girl. Axial CT scan through upper chest inlet area shows symmetrical changes of clavicles at their medial aspects from chronic inflammatory process
There are some similarities between Friedrich’s disease and SAPHO syndrome, a disorder characterized by a variable combination of synovitis, acne, pustulosis, hyperostosis, and osteitis. The different aseptic skin abnormalities are associated with chronic recurrent multifocal osteomyelitis (CRMO), a rare, occasionally symmetrical, nonpurulent inflammation of bone. Although it can involve other bones, the inflammation has a predilection for the anterior chest wall where it can cause tenderness, and swelling. Radiographic abnormalities include sclerosis and periostitis with expansion of the affected bone (Letts et al. 1999).
4 Tumors
Imaging is performed to detect a chest wall mass and to determine its location, size, and character (Garcia-Peña and Barber 2010). When a mass that originates in the chest wall expands into the chest cavity, it forms an obtuse angle with the adjacent chest wall. This feature might be recognized on radiographs, CT, or MRI. Masses that produce rib changes are likely to be extrapleural in location. Conventional radiographs are the first tools for imaging a chest wall mass in most places. Radiographs allow an approximate appreciation of the location of the lesion, its extension, rib destruction, and associated intrathoracic component, pleural effusion, or pulmonary metastases. Further imaging may be required in large or aggressive looking lesions for staging purposes. Both CT and MRI are able to delineate the mass, demonstrate osseous changes, and define the margin and internal structure of the lesion, lymphatic spread, and pleural effusion. Currently, CT is better suited than MRI to show metastases to the lungs. Obviously, when faced with a possibly malignant chest wall mass, an interdisciplinary approach should be used, and the choice of the imaging modality may vary with the availability of, and expertise in using, the local imaging tools.
The chest wall can give rise to a wide variety of benign and malignant tumors that are primarily mesenchymal in origin (Laor 2004), in keeping with the predominant tissue components of the chest wall. Tumors of the chest wall are relatively infrequent during infancy and childhood, but a high proportion is malignant (Kumar et al. 1977; Shamberger et al. 1989; Shamberger and Grier 1994). The tumors often present as a palpable mass, or, less frequently, with pain, cough, or respiratory distress from a large pleural effusion or an extensive intrathoracic component. Secondary involvement of the chest wall from an intrathoracic mass is rare in childhood (Table 1).
Benign lesions | Malignant lesions |
|---|---|
Chondroma | Chondrosarcoma |
Osteochondroma | Osteochondrosarcoma |
Osteoma | Osteosarcoma |
Fibroma | Fibrosarcoma |
Lipoma | Mesenchymal sarcoma |
Eosinophilic granuloma | Ewing’s sarcoma (Askin’s tumor) |
Hemangioma | Rhabdomyosarcoma |
Mesenchymal hamartoma | Leiomyosarcoma |
Aneurysmal bone cyst | Lymphoma |
Fibrous dysplasia |
The tumor may be located within the bones and/or within the soft tissues of the chest wall. A sharply marginated osteolytic lesion usually signifies a slow growing (benign) process; however, differentiation from a malignant lesion is not always possible, and biopsy may be required (Kozlowski et al. 1989). Multifocal Langerhans cell histiocytosis (eosinophilic granuloma) with typical osteolytic lesions of the skull vault or vertebra plana allow a confident clinical diagnosis (Fig. 15).
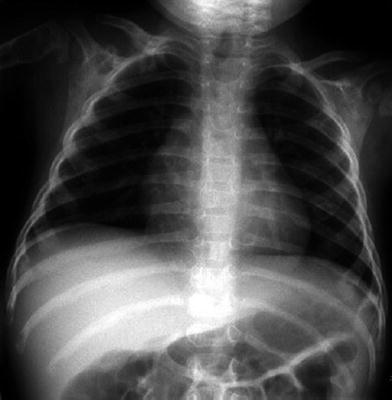

Fig. 15
Langerhans cell histiocytosis in a 14-month-old girl. Chest radiograph shows numerous osteolytic lesions involving almost all ribs, mainly anterior portions, but also scapulae, clavicles, humeri, as well as multiple skeletal parts not shown on this film
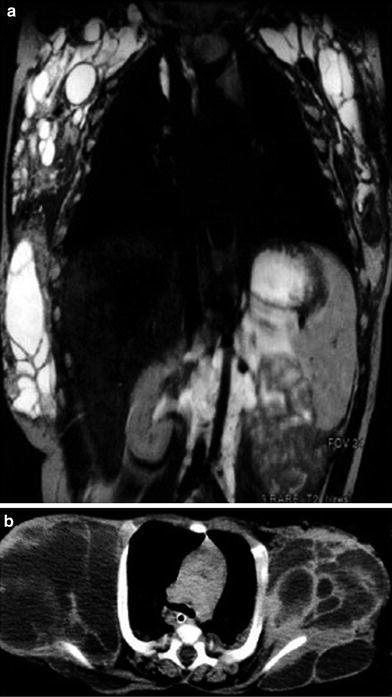
Vascular lesions like hemangioma and vascular malformations are amongst the most common soft tissue tumors of childhood that may be found in the chest wall. They are most prevalent in neonates, infants, and young children. According to the Mulliken and Glowacki classification (Mulliken and Glowacki 1982), hemangiomas are benign proliferating tumors of blood vessels. Infantile hemangiomas typically appear in the first weeks of life, increase rapidly in size during the first year, and subsequently regress gradually until they may disappear completely in early childhood. When located beneath the skin, hemangiomas may exhibit the typical strawberry red color. On US or MRI, hemangiomas are characterized as nonspecific, circumscribed and well-vascularized soft tissue mass with prominent feeding vessels. Vascular malformations are nonneoplastic lesions present at birth and classified based on their vascular composition and amount of blood flow. High-flow malformations include arterial or arterio-venous lesions which can be treated by transcatheter embolization. Low-flow lesions include lymphatic, capillary, venous, or mixed malformations. Lymphatic malformations (Lymphangiomas) are space-occupying, nonneoplastic lesions predominantly composed of lymphatic vessels, and are also called cystic hygromas when dilated lymphatic vessels lead to the formation of large cysts. Lymphangiomas are usually apparent at birth and found in the neck and chest wall. Extension into the mediastinum and axilla can occur (Fig. 16). The growth of lymphangiomas is usually self-limited, but bleeding into the cysts or infection may lead to a sudden increase in size. Venous and capillary malformations have previously also been called cavernous or capillary hemangioma on histopathologic grounds, leading to some confusion in the nomenclature of vascular lesions. As both lymphatic and venous malformations present as mass lesions consisting of multiple fluid-filled spaces, differentiation may only be possible by intravenous contrast administration on CT or MRI. Lymphangiomas will only show contrast enhancement in the walls and septations of macrocysts, or will show diffusely in areas with microcysts (Fig. 17). Venous malformations may contain phlebolites and typically show slow and incomplete enhancement of the vascular spaces on dynamic contrast-enhanced MRI (Fig. 18).