Weight (kg)
Tube current (mAs)
Insp./exp.
Kilovoltage (kV)
<10
40/20
80
10–14
50/25
80
15–24
60/30
80
25–34
70/35
80
35–44
80/40
80
45–54
90/40
90
55–70
100–120/40
100–120
3.3.3 Postprocessing Techniques
Three main currently available postprocessing techniques for evaluation of large airway disorders include 2D, 3D and 4D reconstructions.
3.3.3.1 2D Reconstruction Imaging
Single-voxel-thick 2D multiplanar reformatted (MPR) images can be readily created at the CT console or at a distant postprocessing workstation in any desired plane (e.g., coronal, sagittal, oblique). Curved reformation along the long axis of the large airways may be obtained by drawing a reference line through the airway center on sagittal MPRs (Figs. 1a, 2b). Such reconstruction is invaluable for obtaining accurate airway measurements as are often needed prior to invasive interventions. Thick slab (generally 3–10 mm) or MPR volume reformation may be obtained by adding adjacent thin slices to balance spatial with contrast resolution to the desired level. Minimum intensity projection (MinIP) volume-rendered images, formed by choosing the lowest attenuation voxels, may be obtained to help increase conspicuity of the airways and lung parenchyma (Laya and Lee 2012; Lee and Boiselle 2009; Lee et al. 2011b, 2012, 2013).
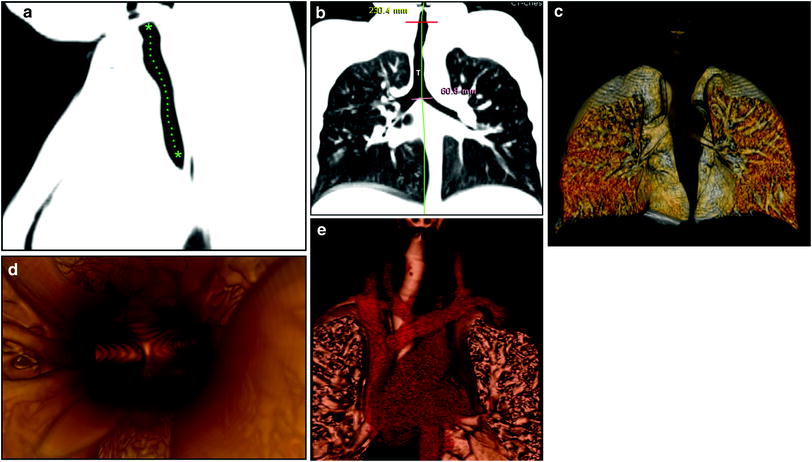
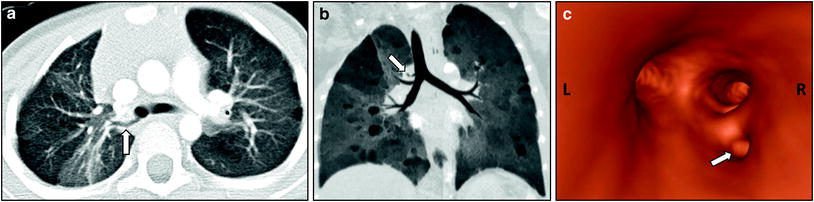

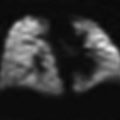
Fig. 1
Normal large airways of a 6-year-old girl, a Sagittal reformatted CT image of the large airways shows a reference position (green asterisks) through the center of the airway for reconstruction of a curved coronal reformatted CT image, b Curved coronal reformatted CT image demonstrates a straighten view of the entire trachea (T), c 3D external volume rendering image (i.e., virtual bronchography) of the large airways, d 3D internal volume rendering (i.e., virtual bronchoscopy) image obtained at the level of carina. Bilateral main stem bronchi are patent, e Combined 3D external volume rendering image of the large airways and surrounding cardiovascular structures (red)

Fig. 2
Tracheal bronchus in a 4-year-old girl who presented with recurrent right upper lobe pneumonia. Axial lung window CT image (a), coronal minimum intensity projection image (b), and 3D virtual bronchoscopy image (c) show an anomalous bronchus (arrow) supplying the posterior segment of the right upper lobe bronchus directly arising from the right lateral wall of the trachea
3.3.3.2 3D Reconstruction Imaging
3D airway reconstruction is typically obtained using volume rendering. This is a computational method that analyzes all available data voxels with an edge detection algorithm to create external and internal renderings of the large airways. External 3D renderings (i.e., virtual bronchography) show the relationship of the outer airway surfaces to nearby structures (Fig. 1c). Internal 3D renderings (i.e., virtual bronchoscopy) provide intraluminal views of the airways on par with conventional (direct) bronchoscopy (Fig. 1d). Virtual bronchoscopy is useful in preoperative planning. Moreover, it may even substitute for traditional diagnostic bronchoscopy, thus potentially avoiding an invasive procedure and the need for general anesthesia (Lee et al. 2012, 2013). For preoperative evaluation of underlying mediastinal vascular anomalies resulting in large airway compression, a combined view of both large airways and vascular structures can provide comprehensive assessment (Fig. 1e).
3.3.3.3 4D Reconstruction Imaging
4D MDCT combines 3D renderings with real-time evaluation, which is considered as the fourth dimension. The large airways are depicted in real-time as a continuously moving 3D image. Innovative 320 MDCT scanners can display the large airways up to a 16-cm-long craniocaudal extent (sufficient even for most older children) with true isometric, isophasic, and isovolumetric 4D imaging throughout the respiratory cycle. 4D techniques are particularly useful for evaluating dynamic airway abnormalities such as TBM, assessing anatomical changes with time (Lee et al. 2012, 2013).
4 Spectrum of Imaging Findings
4.1 Congenital Large Airway Disorders
4.1.1 Non-vascular Congenital Large Airway Disorders
4.1.1.1 Tracheobronchial Branching Anomalies
Ectopic Bronchus: Tracheal, Esophageal, Cardiac Bronchus
Tracheal bronchus, accessory cardiac bronchus (ACB), and esophageal bronchus are the three major congenital tracheobronchial branching anomalies, in descending order of frequency. Tracheal bronchus describes a spectrum of anomalies characterized by an aberrant bronchus originating from the trachea or main bronchi directed toward the upper lobes (Fig. 2). Previously, it was narrowly defined as a right upper lobe bronchus arising from the trachea, hence its eponym “bronchus suis” due to a similar morphology in pigs. ACB refers to an extra bronchus arising from the inner wall of the right main bronchus or bronchus intermedius opposite the right upper lobe bronchus origin and advancing 1–5 cm caudally toward the pericardium, parallel to the bronchus intermedius (Berrocal et al. 2004; Ghaye et al. 2001; Lee et al. 2011b, 2012, 2013). Esophageal bronchus is characterized by a lobar bronchus originating from the esophagus, usually supplying the right lower lobe medial basal segment (Pimpalwar and Hassan 2012; Verma et al. 2008).
Of these tracheobronchial branching anomalies, tracheal bronchus is most common, with a prevalence of 0.1–2 % on the right and 0.3–1 % on the left. The frequency of ACB is 0.08 % (Ghaye et al. 2001). Esophageal bronchus is extremely rare with data limited to case reports. All of these entities may present with recurrent pulmonary infection, although tracheal and accessory cardiac bronchi may be incidental and asymptomatic (Ghaye et al. 2001; McGuinness et al. 1993; Pimpalwar and Hassan 2012; Yildiz et al. 2006). A characteristic history for tracheal bronchus is persistent right upper lobe pneumonia or atelectasis following endotracheal intubation (O’Sullivan et al. 1998).
Chest radiographs occasionally depict the anomalous bronchus but often are normal. They may show consolidation or atelectasis in the portion of the lung supplied by the anomalous bronchus. Barium swallow is diagnostic for esophageal bronchus, establishing the abnormal communication. For all of the entities, MDCT with 2D and 3D reformats accurately characterizes the aberrant bronchus, assisting in preoperative planning, and documents any associated anomalies (Fig. 2b, c). In symptomatic patients, surgical resection of the anomalous bronchus and affected lung tissue is recommended. There is also a potential role for bronchial reimplantation when the aberrant bronchus is detected early but expected to produce substantial future lung damage (Ghaye et al. 2001; McGuinness et al. 1993; Lee et al. 2011b, 2012, 2013; Pimpalwar and Hassan 2012; Verma et al. 2008; Yildiz et al. 2006).
Heterotaxy: Left Isomerism and Right Isomerism
Heterotaxy, or situs ambiguous, refers to an abnormal arrangement and development of the visceral organs. This is in contrast to the normal organ position (situs solitus) or its complete mirror image (situs inversus). While heterotaxy has many variations, there are two major subdivisions: left isomerism (“double left-sidedness”) and right isomerism (“double right-sidedness”). In each condition, there is a relative duplication of the structures normally located on one side of the body on the opposite side. Findings in left isomerism include a midline liver, multiple spleens (polysplenia), bilateral bilobed lungs with hyperarterial bronchi (located below the ipsilateral pulmonary artery), and bilateral pulmonary atria (receiving blood from the pulmonary veins like the normal left atrium) (Fig. 3). Findings in right isomerism include a midline liver, an absent spleen (asplenia), bilateral trilobed lungs with bilateral minor fissures and eparterial bronchi (above the ipsilateral pulmonary artery, and bilateral systemic atria (receiving blood from the inferior vena cava [IVC] like the normal right atrium). Heterotaxy is overall very rare and associated with a variety of anomalies including congenital heart disease in 50–100 % of cases and intestinal malrotation. An interrupted IVC with azygous/hemiazygos continuation is frequently observed in right isomerism but not in left isomerism (Applegate et al. 1999).
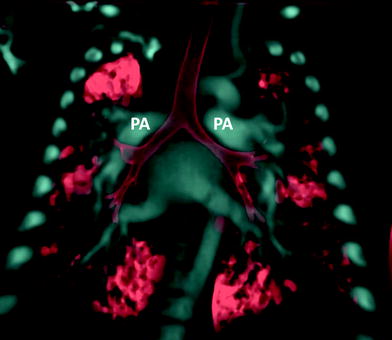

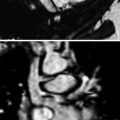
Fig. 3
Heterotaxy patient with hyparterial bronchial in left isomerism. Coronal volume rendered CT image shows both main stem bronchi coursing inferior to their ipsilateral pulmonary artery (PA) on each side
Chest radiography is the first step in diagnosis and is able to establish the position of the aortic arch, cardiac apex, and stomach bubble. If these structures are not located on the left (situs solitus) or the reverse (situs inversus), then heterotaxy is present. The imaging work-up should next extensively confirm and detail the aberrant anatomy and any associated anomalies. Specifically, the position of the atria, venous drainage below the diaphragm relative to midline, aorta relative to midline, stomach, liver, gallbladder, and cardiac apex should be assessed. Additionally, the presence of intestinal malrotation and bilobed versus trilobed lungs as well as number and appearance of spleens should be documented. For these purposes, a variety of imaging modalities may be utilized including upper gastrointestinal (GI) series, ultrasound/echocardiography, CT, MRI, and when necessary angiography (Applegate et al. 1999). Treatment and prognosis depend on the extent and severity of anomalies. Patients with left isomerism generally have less severe cardiac defects than do patients with right isomerism with better chance of surgical repair (Kim 2011).
4.1.1.2 Congenital Tracheal Stenosis
Congenital tracheal stenosis (CTS) is a rare anomaly in which focal or diffuse complete tracheal cartilage rings result in deficient or absent tracheal membranes, causing fixed tracheal narrowing (Lee et al. 2011b, 2012, 2013; Lee and Siegel 2007). There are three major types: generalized total tracheal hypoplasia but normal bronchi (type I); funnel-like stenosis and gradual airway tapering with a normal subglottic but stenotic carinal end of the trachea (type II); and short-segment airway stenosis, approximately 2–5 cm in length (type III). These variations occur in 30, 20, and 50 % of cases, respectively (Cantrell and Guild 1964; Herrera et al. 2007; Lee et al. 2011b, 2013). Concurrent cardiovascular defects occur in most patients, the most common being left pulmonary artery sling (Lee et al. 2012; Antón-Pacheco et al. 2012). Patients present typically by age 1 with recurrent pneumonia, wheezing, and biphasic stridor (Lee et al. 2011b).
Chest radiography and fluoroscopy may show tracheal narrowing but are often insensitive. MDCT with 2D and 3D reformations is superior to chest radiograph and fluoroscopy for accurate detection and characterization (Fig. 4). CT precisely depicts the location and extent of stenosis and can also evaluate for other congenital anomalies. CT virtual bronchoscopy closely mirrors images from conventional bronchoscopy and may in the future be sufficient for diagnosis (Herrera et al. 2007; Lee et al. 2011b, 2012, 2013; Lee and Siegel 2007).
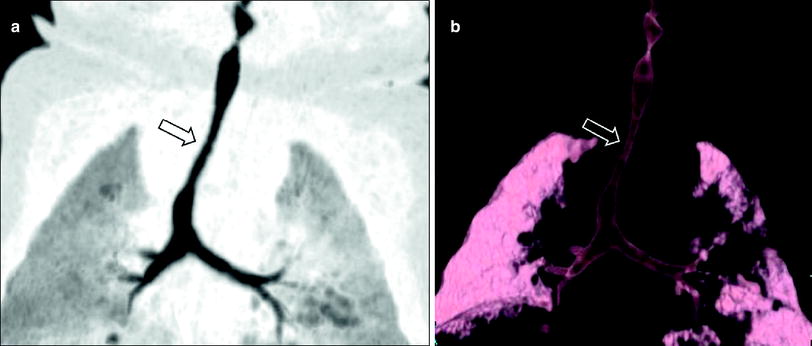

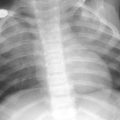
Fig. 4
Infant girl with congenital long segment tracheal stenosis who presented with severe respiratory distress. Coronal minimum intensity projection image (a) and 3D external volume rendered image (b) shows an approximately 2 cm long segment concentric stenosis (arrow) of the trachea. At bronchoscopy, multiple complete tracheal rings in the region of the tracheal narrowing seen on CT images were identified
In symptomatic patients, CTS is managed surgically when feasible. Short stenoses (≤5 cm) are treated with segmental resection and end-to-end anastomosis. Longer stenoses were previously treated with patch or tracheal autograft repair but now slide tracheoplasty is preferred (Antón-Pacheco et al. 2012; Herrera et al. 2007; Lee et al. 2012).
4.1.1.3 Pulmonary Agenesis, Aplasia, and Hypoplasia
This spectrum of rare congenital disorders, referred to as the agenesis-aplasia-hypoplasia complex, is characterized by large airway underdevelopment. Pulmonary hypoplasia, generally caused by lesions that preclude normal lung growth, is the least severe form, characterized by bronchial, lung, and pulmonary artery hypoplasia. Pulmonary aplasia is intermediate severity, with absence of the lung and pulmonary artery, but a preserved although primitive main bronchus. Pulmonary agenesis is the most severe with total absence of the bronchus, lung, and pulmonary artery (Lee 2007; Lee et al. 2008a, 2010a , b, c , 2011a, b, 2012, 2013) (Fig. 5). Associated cardiovascular, gastrointestinal, or skeletal anomalies are seen in >50 % of cases (Biyyam et al. 2010). Affected pediatric patients may be asymptomatic or present with recurrent pulmonary infection, cough, and dyspnea (Lee et al. 2008a, 2010a , b, c , 2011b, 2013).
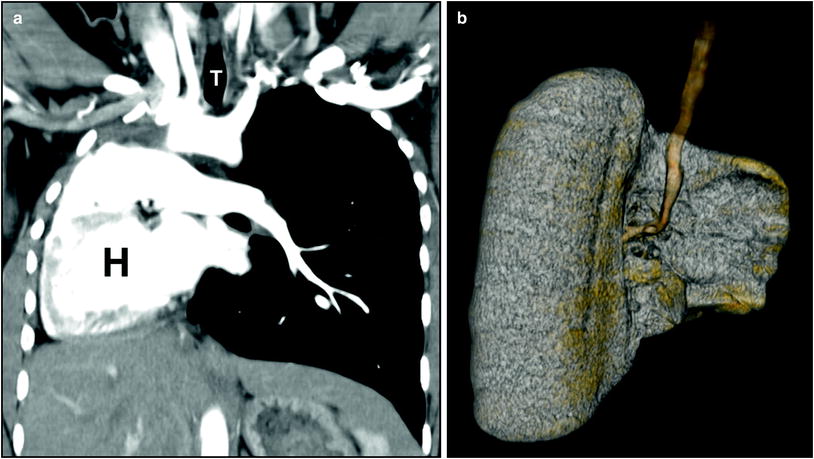

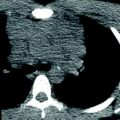
Fig. 5
Right lung agenesis in a 16-year-old girl who presented with respiratory distress and abnormal chest radiograph. CT was subsequently obtained for further evaluation. a Enhanced coronal CT image shows the entire mediastinal structure shifted to the right side of hemithorax. H heart. T trachea, b 3D volume rendered image of the large airway from the posterior view demonstrates absent right bronchus and right lung. Compensatory hypertrophy and hyperinflation of the left lung is also seen
Radiographs show widespread opacification of the affected hemithorax with ipsilateral shift of the mediastinum (Biyyam et al. 2010). CT with 2D, 3D, and 4D reformats accurately characterizes the extent of hypoplasia or aplasia of the pulmonary arteries, airways, and lungs. In symptomatic pediatric patients for whom surgical correction is indicated, CT is ideal for preoperative planning (Lee et al. 2008a, 2010a , b, c , 2011b, 2012, 2013).
4.1.2 Vascular Congenital Large Airway Disorders
Many mediastinal vascular anomalies cause symptomatic extrinsic compression of the large airways. They may be broadly classified into innominate artery compression syndrome (detailed below), vascular rings (vascular and ligamentous structures that encircle the esophagus and trachea), and vascular slings (typified by pulmonary artery sling) (Hernanz-Schulman 2005; Lee et al. 2013). In general, the imaging work-up begins with chest radiography to discern the laterality or bilaterality of the aortic arch and then barium esophagram to determine if there is any abnormal impression on the trachea and/or esophagus. A normal esophagram generally excludes a vascular ring or sling but if abnormal, further evaluation with MDCT (or MRI) is often pursued to better characterize the anomaly particularly before surgical ligation (Hernanz-Schulman 2005).
4.1.2.1 Innominate Artery Compression Syndrome
In this disorder, there is an anomalous innominate artery that originates on the left side of the aortic arch and courses obliquely from left to right, compressing the anterior trachea. Patients presents with variable respiratory distress ranging from mild stridor to near-death episodes. The classic appearance on barium swallow is anterior tracheal compression without esophageal compression. Pulsatile anterior tracheal compression seen on bronchoscopy should heighten suspicion and prompt CT for accurate characterization (Fig. 6). Mild symptoms usually resolve by age 2. In more severe cases, surgical correction with inominopexy or aortopexy (suspension of the innominate artery or aorta in front of the sternum, respectively) may be necessary (Lee et al. 2010a, 2011b, 2012, 2013).
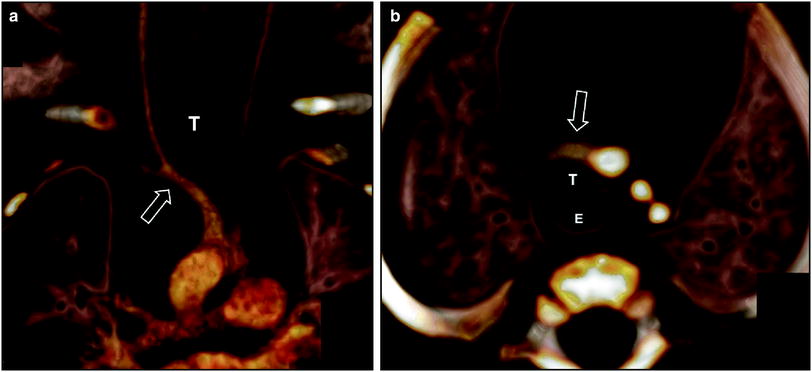

Fig. 6
Innominate artery compression syndrome in a 1-month old boy with stridor, respiratory distress, and acute life threatening event. Frontal view (a) and superior view (b) of the 3D volume rendered images of the large airways and vascular structures show the compression of the trachea (T) by an innominate artery (arrow). E esophagus
4.1.2.2 Double Aortic Arch
The most symptomatic cause of vascular tracheoesophageal compression, double aortic arch is a true vascular ring in which persistent bilateral aortic arches encircle the trachea and esophagus. Secondary tracheomalacia due to extrinsic compression also occurs. Patients present with cough, wheezing, stridor, and dysphagia. On barium swallow, there is anterior tracheal compression and posterior esophageal compression (Fig. 7a). MRI and MDCT accurately depicts the anomaly and identifies the dominant arch, essential information in presurgical planning (Hernanz-Schulman 2005; Lee et al. 2010a, 2011b, 2012, 2013; Kondrachuk et al. 2012) (Fig. 7b).
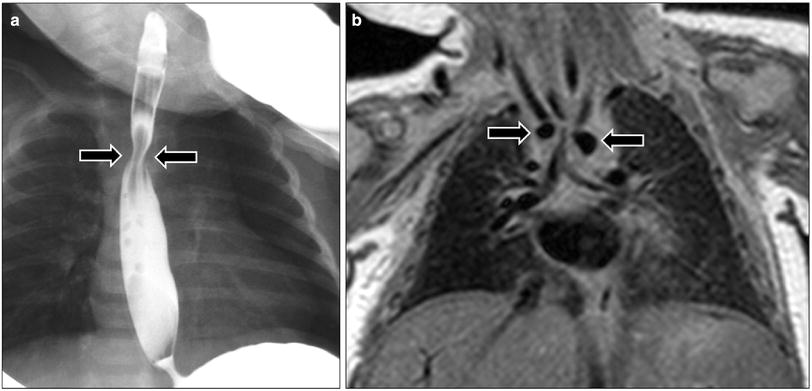

Fig. 7
Double aortic arch in a 1-month-old girl who presented with respiratory distress and feeding difficulty. a Frontal radiograph obtained during barium swallow study shows a narrowing (arrows) in the upper esophagus. b Coronal proton density MR image shows two aortic arches (arrows) consistent with double aortic arch with tracheal narrowing at this level
4.1.2.3 Right Aortic Arch with Aberrant Left Subclavian Artery
This anomaly is also a vascular ring characterized by a right aortic arch and posterior left subclavian artery originating from a diverticulum of Kommerell (equivalent to a left arch remnant). In 90 % of cases, there is a left-sided ductal ligament arising from the left pulmonary artery that completes the ring (Hernanz-Schulman 2005). As in double aortic arch, symptoms include dysphagia and/or respiratory distress, and secondary tracheomalacia may occur (Lee et al. 2013). On barium swallow, there is a posterior impression on the esophagus only. MDCT provides precise assessment in anticipation of surgical correction (Hernanz-Schulman 2005; Lee et al. 2010a, 2011b, 2012, 2013; Kondrachuk et al. 2012).
4.1.2.4 Pulmonary Artery Sling
Pulmonary sling is a rare disorder characterized by an anomalous origin of the left pulmonary artery from the right pulmonary artery. The aberrant left main pulmonary artery travels to the left chest between the trachea and esophagus (Fig. 8). Presenting symptoms include stridor, hypoxia, and apneic episodes. On barium swallow, there is posterior tracheal compression and anterior esophageal compression. MDCT demonstrates not only the primary anomaly in anticipation of surgical correction but also commonly associated malformations of the airway and/or heart (Lee et al. 2010a, 2011b, 2012, 2013; Kondrachuk et al. 2012).
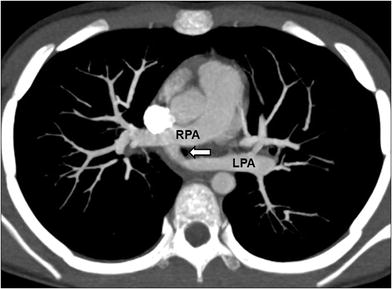

Fig. 8
Pulmonary artery sling in an 8-year-old girl who presented with chronic respiratory distress. Axial maximum intensity projection CT image shows an anomalous left main pulmonary artery (LPA) arising from the right main pulmonary artery (RPA). Tracheal compression (arrow) by the anomalous left pulmonary artery is seen at this level
4.1.2.5 Tetralogy of Fallot with Absent Pulmonary Valve
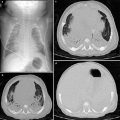
This congenital disorder combines the usual features of tetralogy of Fallot (TOF) (overriding aorta, pulmonic stenosis, right ventricular hypertrophy, and an anterior ventricular septal defect [VSD]) with absence of the pulmonary valve (APVS). It accounts for 3–6 % of TOF cases. Affected pediatric patients develop aneurismal dilation of the main and branch pulmonary arteries leading to secondary large airway narrowing and/or tracheobronchomalacia. Up to half present in infancy with variable respiratory distress requiring mechanical ventilation in severe cases. The rest are clinically indistinguishable from other TOF patients and may experience cyanotic episodes and/or congestive heart failure. On chest radiography, the characteristic “boot-shaped” heart of TOF is often not present because the dilated pulmonary arteries overlap with the left heart border. While echocardiography is the major imaging modality for all TOF patients, CT is an important tool for preoperative planning in TOF-APVS (Kirshbom and Kogon 2004; Vincenti et al. 2012) (Fig. 9).
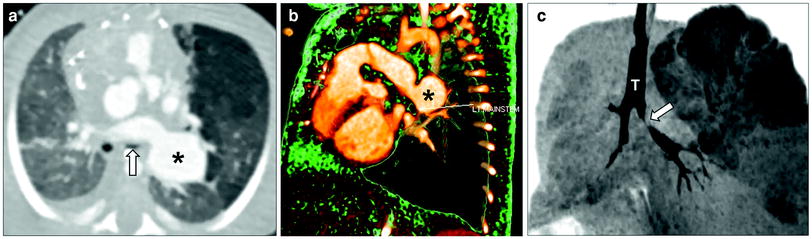

Fig. 9
Tetralogy of Fallot with absent pulmonary valve and aneurysmal dilatation of left pulmonary artery in a neonate boy who presented with severe respiratory distress. Axial lung window CT image (a), 3D volume rendered image (b), and minimum intensity projection image (c) show an aneurysmal dilatation of the left pulmonary artery (asterisk) compressing and inferiorly displacing the left upper lobe bronchus (arrow). Also noted is air-trapping in the left upper lobe best seen on minimum intensity projecting image (c). T trachea
4.1.3 Dynamic Congenital Large Airway Disorder
4.1.3.1 Tracheobronchomalacia
Tracheobronchomalacia (TBM) is characterized by abnormal collapse of the trachea or bronchi on expiration due to airway wall softening, supporting cartilage weakening, and/or supporting muscle hypotonia. It may be congenital (primary) or acquired (secondary) due to such causes as prior infection, surgery, or extrinsic mediastinal vascular compression (as previously discussed) (Laya and Lee 2012; Lee and Boiselle 2009; Lee et al. 2008b, c, 2009, 2010a, b, c, 2011b, 2012, 2013). TBM may be associated with cardiac anomalies, bronchopulmonary dysplasia, gastroesophageal reflux, and neurologic problems (Carden et al. 2005). Presenting symptoms, exacerbated during forced expiration, or crying, include cough, wheezing, stridor, dyspnea, cyanosis, and recurrent respiratory infections (Laya and Lee 2012).
Whether diagnosed by bronchoscopy or imaging, TBM is defined as >50 % airway collapse on expiration. Noninvasive evaluation historically was limited to chest radiography and airway fluoroscopy (Fig. 10). MDCT with multiplanar 2D and 3D reformats has now become the gold standard, combining superior anatomical detail with the ability to perform quantitative measurements (Fig. 11). Additionally, it can also detect air-trapping and any associated anomalies often detected in pediatric patients with TBM. Most often performed is paired inspiratory–expiratory MDCT. Notably, on expiration the tube current may be reduced by half without compromising diagnostic ability (Lee et al. 2010a, b, c). Real-time dynamic 4D assessment is also now possible with cine 64-MDCT and true 4D imaging with the 320-MDCT (Laya and Lee 2012; Lee and Boiselle 2009; Lee et al. 2008b, c, 2009, 2010a, b, c, 2011b, 2012, 2013).
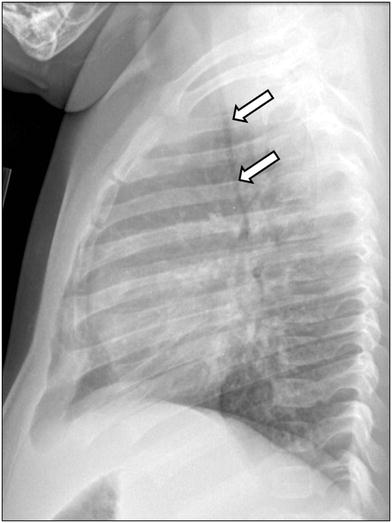
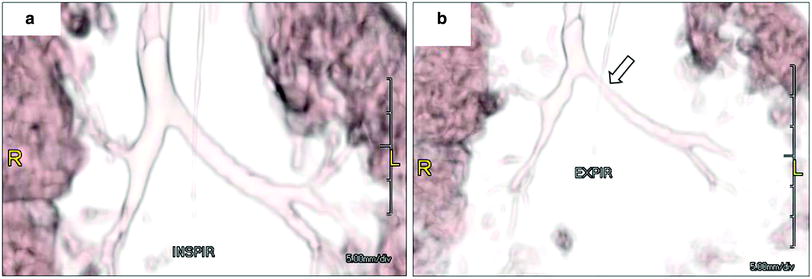

Fig. 10
Tracheomalacia in an infant girl who presented with respiratory distress and wheezing. Lateral chest radiograph obtained at end expiration shows markedly decreased caliber of the intrathoracic trachea (arrows)

Fig. 11
Bronchomalacia in a 4-month-old ex-25 week premature infant with chronic lung disease and tracheobronchomalacia. 3D volume rendered images of the large airways obtained at end inspiration (a) and at end expiration (b) show decrease (>50 %) in caliber of the bronchial tree, particularly proximal left main stem bronchus (arrow) as well as the left upper lobe and right upper lobe bronchi
Mild to moderate TBM symptoms may substantially improve or even completely abate because the tracheal cartilage grows stronger with age. Conservative measures include pulmonary physiotherapy, humidified oxygen, and antibiotics for concomitant pulmonary infections. In patients with more severe disease, options may include continuous positive airway pressure (CPAP) tracheostomy, airway stenting, and surgical tracheoplasty or aortopexy (Carden et al. 2005; Laya and Lee 2012; Lee et al. 2012; Lee and Boiselle 2009).
4.2 Acquired Large Airway Disorders
4.2.1 Infectious Large Airway Disorders
4.2.1.1 Croup
A common cause of acute pediatric airway obstruction, croup is characterized by diffuse laryngeal and tracheal inflammation as well as severe subglottic laryngeal swelling and narrowing. It may be caused directly by a virus (typically parainfluenza virus 1) or an allergic inflammatory response to a virus. Patients generally 7 months to 3-years old present with barking cough, inspiratory stridor, and hoarseness (Currarino and Williams 1982; Chapman et al. 2012; Cherry 2008).
The classic appearance on AP neck radiographs is termed the “steeple sign” with loss of the normal shouldering edges of the subglottic airway with tapered narrowing to the level of the glottis (Fig. 12). On the lateral view, the normally sharp margins of the subglottic airway are obliterated. Also characteristic are distention of the hypopharynx and larynx and cervical tracheal narrowing that improve or even resolve with expiration. It should be cautioned that 50 % of neck radiographs in croup are normal (Currarino and Williams 1982; Chapman et al. 2012; Salour 2000; Huang and Shih 2012). Higher-level imaging is only pursued if more complex pathology is anticipated.
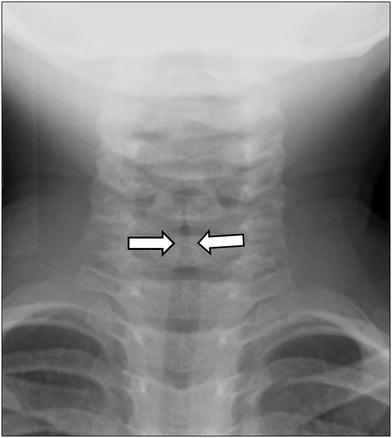

Fig. 12
Croup in a 1-year old boy who presented with barking cough. Anteroposterior radiograph shows a steeple appearance of the subglottic trachea due to symmetric subglottic narrowing (arrows) with loss of the normal shoulders of the upper airway
Croup is generally self-limited. Medical management is tailored to the severity of disease. Options include corticosteroids, nebulized or L-epinephrine, and heliox therapy (Pitluk et al. 2011).
4.2.1.2 Bacterial Tracheitis
This large airway disorder is characterized by potentially life-threatening acute airway obstruction due to thick, adherent tracheal membranes. Previously, Staphylococcus aureus was the most common causative pathogen but now many strains of bacteria are implicated. Patients generally 3–8 years old present with cough and stridor with subsequent hoarseness, fever, and tachypnea, often rapidly declining despite medical therapy (Miranda et al. 2011; Shargorodsky et al. 2010).
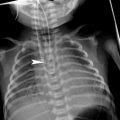
Neck and chest radiographs demonstrate subglottic tracheal narrowing and contour irregularity of the proximal trachea (Fig. 13). Characteristic are tracheal membranes, typically linear, which may detach and resemble foreign bodies (Han et al. 1979; Sammer and Pruthi 2010). Pneumomediastinum is a rare radiographic presentation (Hedlund et al. 1998).
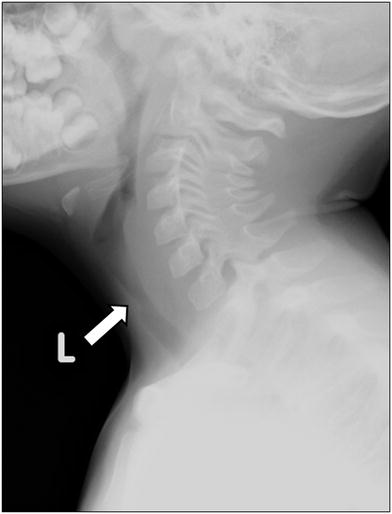

Fig. 13
Bacterial tracheitis in a 5-year-old boy who presented with cough, stridor and fever. Lateral soft tissue neck radiograph shows a subglottic tracheal narrowing and contour irregularity (arrow)
In managing bacterial tracheitis, there should be a low threshold to perform endotracheal intubation or establish a surgical airway due to the high risk of airway compromise. Broad-spectrum IV antibiotics and often corticosteroids are administered. However, most crucial are rigid laryngoscopy and bronchoscopy allowing direct debridement of purulent debris as well as sampling for microbiological culture (Miranda et al. 2011; Shargorodsky et al. 2010).
4.2.1.3 Epiglottitis
Epiglottitis is a relatively rare bacterial infection of the epiglottis and surrounding structures (aryepiglottic folds, arytenoids, and supraglottic larynx) resulting in potentially life-threatening acute airway obstruction. Prior to vaccination against Haemophilus influenza type b (Hib), previously the most common causative pathogen, the disease presented in children ages 2–5 years old with drooling, dysphagia, dyspnea, and dysphonia (“4 Ds”) and high fever. Since vaccination became available, the incidence has dropped 40-fold, and children present at a mean age of approximately 12-years old with milder symptoms such as low-grade fever and croup-like cough (Chapman et al. 2012; John and Swischuk 1992; Wheeler et al. 2008).
Characteristic findings on neck radiography are marked thickening of the epiglottis and aryepiglottic folds (Chapman et al. 2012; John and Swischuk 1992). The impression of the inflamed epiglottis on the airway known as the “thumbprint sign” is classic (Grover 2011; Podgore and Bass 1976). The frontal neck radiograph may show a funnel or steeple configuration of the glottic and subglottic airway (John and Swischuk 1992). The omega epiglottis, a normal variant in which the epiglottis is uniformly thickened with a horseshoe shape, may mimic epiglottitis. However, in true epiglottitis the aryepiglottic folds are thickened (Chapman et al. 2012).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree