Fig. 1
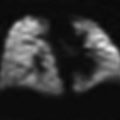
Transverse US scan of the right side shows several comet tails adjacent to the diaphragm (arrows), indicative of normal aerated lung
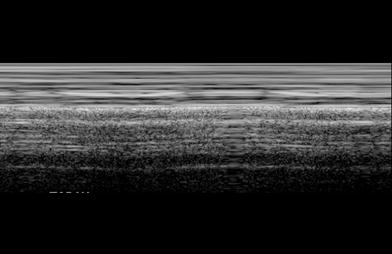
Fig. 2
“Seashore sign” produced by lung motion, seen on transverse intercostal M-mode US scan
The main artifacts seen on US study of the aerated lung base are the so-called mirror image artifacts, caused by sound wave reflection when the ultrasound beam strikes the diaphragm. Depending on the angle at which the US beam is directed during transdiaphragmatic scanning of the lung base, a dual image of the liver or spleen can be observed above the diaphragm, simulating a parenchymal consolidation. This artifact, known as pseudo-consolidation (Ben-Ami et al. 1993), is indicative of normal air-filled lung. Mirroring of the image can also occur when using color Doppler, and results in duplication of vascular structures (Fig. 3).
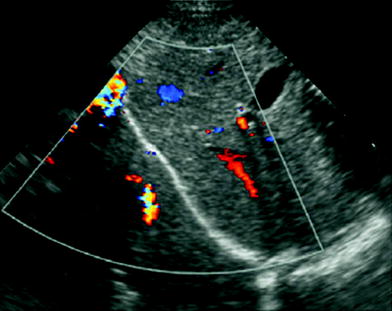

Fig. 3
Mirror image artifact. Transdiaphragmatic longitudinal US scan in a 2-month-old boy shows “duplication” of the liver and its Doppler signals, projecting over the right lung base
3.2 Congenital Malformations
In countries where obstetric sonography is routinely performed, the detection rate of congenital lung malformations has risen considerably (Davenport et al. 2004). The fetal lung can be easily identified on prenatal US studies using a transverse four-chamber view of the fetal chest, a routine component of obstetric ultrasound protocols. It is seen as a solid structure of medium-level echogenicity that varies slightly throughout gestation. This US appearance is produced by the combination of the lung water content and parenchymal network of bronchial, vascular, and mesenchymal elements. Furthermore, lung vascularization can be readily assessed by pulsed, color, or power Doppler (Fig. 4).
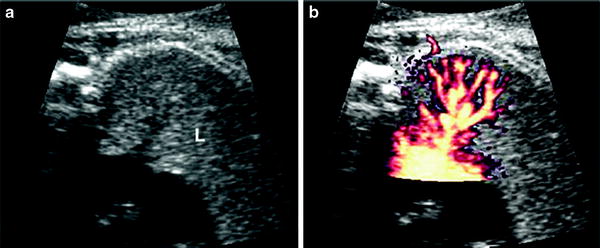

Fig. 4
US scan of normal fetal lung at 23 weeks’gestational age a Four-chamber view shows the lung as a solid medium-level echogenic structure (L) b Pulmonary vessels and their ramifcations are very well seen on Power Doppler. Courtesy of Dr. Edgar Hernández, Ph. D. Clinic Hospital Barcelona
Fetal congenital lung malformations present as areas of abnormal echogenicity exerting a mass effect on adjacent structures. They are hyperechoic with respect to normal lung parenchyma and may be either homogenous or with coexisting cysts. The size of the lesion may be overestimated since the compressed, unaffected lung can acquire echogenicity similar to that of the lesion and be considered a part of it. US is a sensitive technique for detecting congenital lung malformations, but is less reliable for establishing a specific diagnosis, since a similar echo pattern can be seen in many of these conditions.
Several classifications and terms are in use to describe bronchopulmonary malformations, which are now believed to represent a spectrum of anomalies that result from intrauterine airway obstruction (Langston et al. 2003; Correia-Pinto et al. 2010) These malformations include congenital pulmonary airway malformation (CPAM), pulmonary sequestration (PS), bronchogenic cyst (BC), bronchial atresia (BA), and congenital lobar overinflation (CLO). Additional anomalies such as pulmonary agenesis, tracheal bronchus, esophageal and tracheal atresia, and tracheoesophageal fistula are recognized as a part of the spectrum by some authors (Newman et al. 2006). The type of malformation and its sonographic appearance depend on the level and timing of the obstruction, with the vascular abnormality being an associated feature.
3.2.1 Congenital Pulmonary Airway Malformation
Congenital pulmonary airway malformation, previously known as congenital cystic adenomatoid malformation, accounts for 30–47 % of all fetal lung masses detected by US. CPAM is a hamartomatous malformation of the lung with abnormal branching of immature bronchioles that communicate with the tracheobronchial tree (Epelman et al. 2010; Correia-Pinto et al. 2010). Sonographic findings vary depending on the type of malformation (May et al. 1993). Stocker et al. initially classified CPAM into three histologic types (Stocker et al. 1977), which were later expanded into five types (Stocker 1994). The first three types are the forms most often recognized on US. Type 1, the most common, appears on US as single or multiple large cysts, often affecting the entire pulmonary lobe. Type 2 is seen as an echogenic mass with numerous small cysts. Type 3 malformations appear as homogenous echogenic masses without cysts.
Sonographic differentiation between CPAM and pulmonary sequestration may not always be possible. A systemic vessel arising from the aorta has also been described in patients with pulmonary airway malformation (Winters et al. 1997). Furthermore, hybrid lesions are often seen, consisting of CPAM associated with pulmonary sequestration in the same malformation; these represented around 50 % of cases in a pathologic series (Conran and Stocker 1999). Postnatal US study of CPAM is challenging. Since the abnormal lung communicates with the airways, the cysts, which contain fluid during fetal life, fill with air at birth, making their identification difficult on US. The air-filled cluster of cysts is seen as an area of increased echogenicity with a banded appearance (Fig. 5). This US finding, which is known as the “aurora sign,” has also been reported in several acquired interstitial lung diseases (Kohzaki et al. 2003). Therefore, CT and MRI are more suitable for postnatal study of the internal components of this malformation. Postnatal radiography, CT or MRI can be performed immediately if the patient is symptomatic or months later if asymptomatic to determine whether surgery is required.
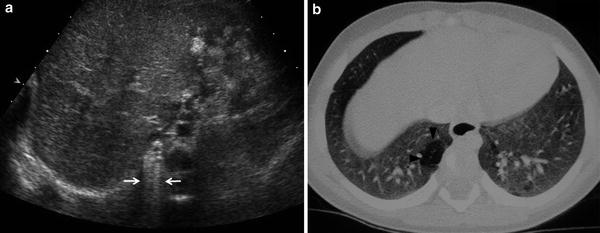

Fig. 5
Postnatal studies in a newborn with prenatal diagnosis of congenital right lung malformation. a Transverse US scan shows a hyperechogenic area with evident B lines in the right hemithorax (arrows). b High-resolution, contrast-enhanced CT in the same patient confirmed the presence of a hyperlucent lesion (arrowhead) corresponding to CPAM
3.2.2 Bronchopulmonary Sequestration
Bronchopulmonary sequestration is a congenital malformation consisting of nonfunctioning lung tissue, which lacks a normal connection to the tracheobronchial tree and is usually supplied by a systemic artery that arises from the thoracic or abdominal aorta. It is the second most common cause of a fetal chest mass, and two different types are classically recognized: intralobar and extralobar. Intralobar sequestration has been found to occur in older infants and some authors have considered it an acquired lesion (Frazier et al. 1997). Extralobar sequestration has its own pleural covering, and venous drainage is into the systemic circulation (azygos-hemiazygos system, portal vein, or inferior vena cava). It has been considered a congenital condition. (May et al. 1993; Ko et al. 2000). Currently, this categorical classification is not universally accepted since mixed systemic and pulmonary venous drainage has been observed in several cases of extralobar sequestration. (Pumberger et al. 2003). Moreover, the intralobar type is also found in neonates and is now considered a congenital rather than an acquired lesion (Newman 2006).
On US study, sequestration is seen as a homogenous or heterogenous echogenic mass usually located in the lower pulmonary lobes, but sometimes occurring at or below the diaphragm. We recommend a subxiphoid approach to study the mass, search for an anomalous feeding vessel arising from the aorta, and investigate the systemic or pulmonary venous drainage (Fig. 6). The azygos may be enlarged in cases of systemic drainage to the azygos-hemiazygos system (Ko et al. 2000). Pulmonary venous drainage is better delineated by CT or MRI than by US (Fig. 7).
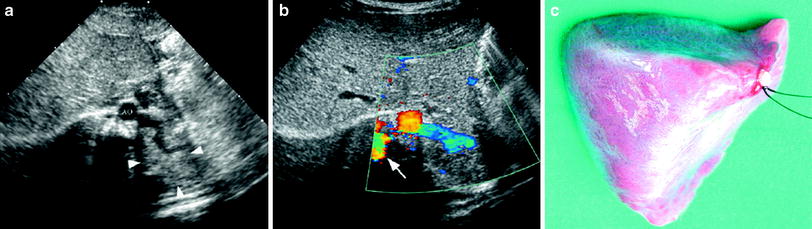
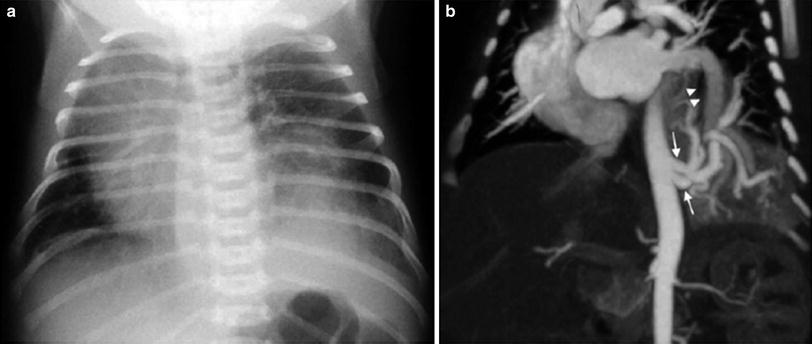

Fig. 6
Extralobar solid sequestration in a 2-month-old boy a In this subxiphoid transverse scan, an echogenic mass (arrowheads) can be seen behind the left lobe of the liver. A tortuous vessel, likely to be a persistent primitive post-brachial artery, is seen arising from the aorta (AO) b Color Doppler shows the abnormal vessel originating at the aorta and supplying the mass. Venous drainage is inferred to be through the enlarged azygos (arrow) c Photo of the surgical specimen of another patient with extralobar sequestration showing its triangular shape and the hiliar vascular nutrition

Fig. 7
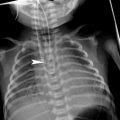
Intralobar sequestration in a newborn boy a Chest X-ray shows opacification of the left lung base b CT angiography demonstrates several systemic vessels arising from the aorta supplying the sequestration (arrows). A large vein is seen draining into the left atrium (arrowheads)
Solid pulmonary sequestrations, particularly those in infradiaphragmatic locations, should be differentiated from congenital neuroblastoma and adrenal hematomas (Manson and Daneman 2001; Langston et al. 2003; Vijayaraghavan et al. 2003). The presence of calcifications (common in neuroblastoma and exceedingly rare in neonatal sequestration) as well as the obstetric history (sequestration is detected in the second trimester and neuroblastoma in the third trimester) are the main distinguishing factors (Fig. 8).
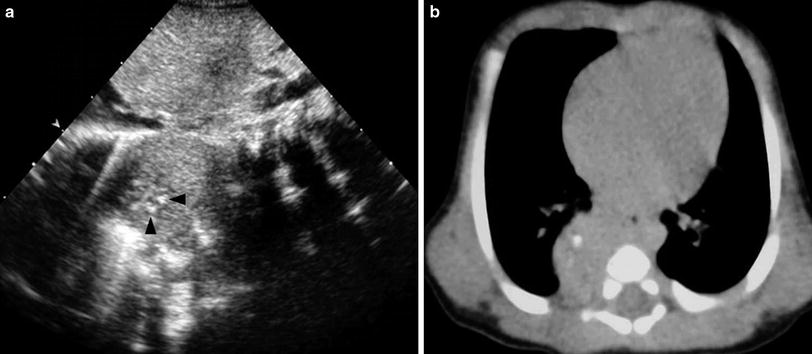

Fig. 8
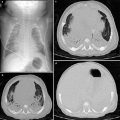
Postnatal US and CT of congenital neuroblastoma. a Longitudinal US scan shows a solid mass in the right paravertebral space with several echogenic foci (arrowheads) representing calcifications. b Unenhanced high-resolution CT confirms the presence of calcifications within the mass. The lesion was detected prenatally in the third trimester of gestation
3.2.3 Lobar Overinflation
Lobar overinflation is characterized by hyperinflation of one part of the lung (usually the apical and posterior segments of the left upper lobe) and is seen on US as an area of homogenous hyperechogenicity with normal pulmonary supply and an absence of cysts (Daltro et al. 2010). The increased echogenicity is believed to be secondary to fluid accumulation in the affected lung (Pariente et al. 2009). Differentiation between CLO and a microcystic form of CPAM or PS may be very difficult before birth. Diagnosis can be confirmed postnatally by chest X-ray and CT imaging (Seo et al. 2006).
3.2.4 Bronchogenic Cyst
Bronchogenic cysts are usually located in the subcarinal region, but they may also be found within the pulmonary parenchyma. Depending on their content, intrapulmonary cysts are seen as unilocular anechoic or weakly echogenic lesions. US is particularly helpful in cases where CT is inconclusive regarding the solid or cystic nature of the lesion (Fig. 9).
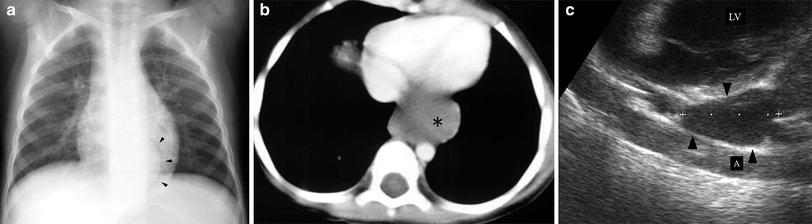

Fig. 9
Bronchogenic cyst in a 4-year-old boy. a Chest plain film shows a curved left paravertebral mass (arrowheads). b On enhanced high resolution CT, a mass of uncertain nature is seen on the left side (asterisk) c Sagittal US scan reveals the cystic characteristics of the lesion (arrowhead). LV left ventricle; A aorta
3.3 Management of Congenital Malformations
Some congenital lung malformations completely or partially involute in the third trimester of gestation. When a huge mass displaces the mediastinum, prenatal interventions such as drainage of the largest cysts in cystic CAPM can be performed. The postnatal management of antenatally diagnosed lung malformations is highly controversial since most newborns are asymptomatic. (Aziz et al. 2004; Laberge et al. 2004). Reports in the literature tend toward conservative management of solid pulmonary sequestration since this malformation may regress spontaneously (García-Peña et al. 1998) (Fig. 10). Nonetheless, resection of cystic or solid/cystic malformations is still advocated owing to the risk of infection and potential malignant transformation to rhabdomyosarcoma or pulmonary blastoma (Papagiannopoulus et al. 2001; Hasiotou et al. 2004); however, the precise timing of surgery remains to be established. Further prospective studies with sufficient follow-up are required for the pros and cons of conservative versus surgical management to be assessed.
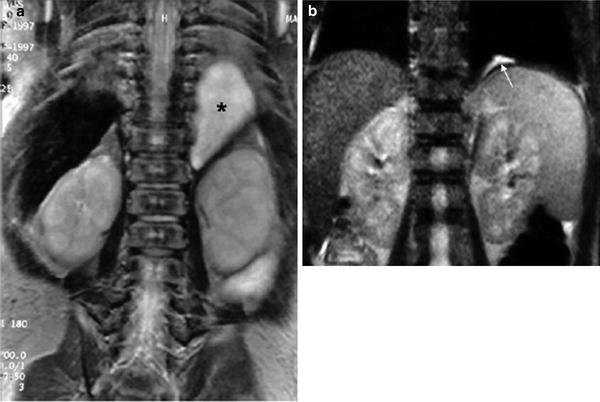

Fig. 10
Involution of prenatally diagnosed pulmonary sequestration a Neonatal MRI coronal view demonstrates a homogeneous solid mass in the left juxtaphrenic region (*) b MRI performed 2 years later shows a small remnant of the lesion (arrow)
3.4 Lung Consolidation
Lung consolidation is the term applied to a lung parenchyma pattern in which pulmonary air is decreased or absent, as occurs in pneumonic consolidation and atelectasis. Pneumonic consolidation refers to filling of the normal air spaces with fluid and inflammatory cells, thereby converting the reflective lung into a solid structure with echogenicity similar to liver (lung hepatization). The air-filled bronchi in the consolidated lung are seen as echogenic branching linear structures converging toward the lung root. This feature is known as the sonographic air bronchogram (Weinberg et al. 1986; Acunas et al. 1989; Yang et al. 1992; Seibert et al. 1998; Kim et al. 2000, Lichtenstein et al. 2009) and is equivalent to the air bronchogram observed on chest X-rays (Fig. 11). The loss of lung volume in atelectasis produces characteristic crowding of the air-filled bronchi and pulmonary vessels (Fig. 12) (Weinberg et al. 1986). In our experience, sonobronchograms are often visualized in patients in whom air bronchograms were not seen on X-rays, a fact that makes US a useful technique for clarifying inconclusive plain film findings (Fig. 13).
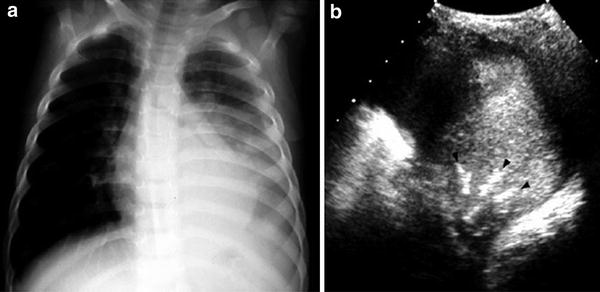
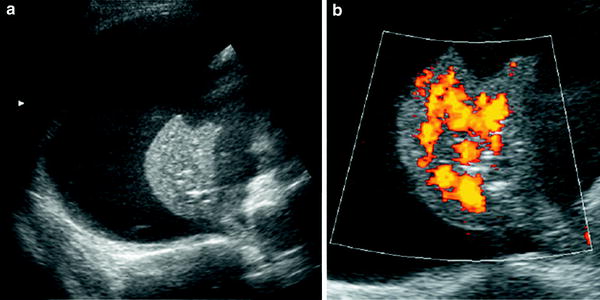
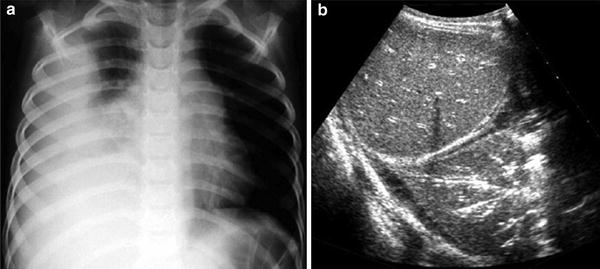

Fig. 11
A 6-year-old girl with left-sided pneumonia and pleural effusion a Increased opacity of the left lung base associated with pleural fluid is visible on the chest radiograph b Intercostal axial US scan with the patient in a right decubitus position shows multiple bright, linear, branching structures (arrowheads), corresponding to air sonobronchograms

Fig. 12
A 10-year-old boy with pleural effusion and secondary atelectasis of the ipsilateral lung a Transverse US scan of the right hemithorax shows profuse pleural fluid and atelectatic lung b Crowded pulmonary vessels, characteristic of pulmonary collapse, are demonstrated by power Doppler. This US finding explains why atelectasis is seen as a hyperintense lesion on CT

Fig. 13
Numerous sonobronchograms not visualized on the chest plain film a Chest radiograph of a 4-year-old boy shows almost complete opacification of the right hemithorax. No airbronchograms are identified, hence differentiation between consolidation and pleural fluid is not possible b Subcostal US view shows that the opacification corresponds to a pulmonary consolidation with a huge sonobronchograms
In patients with asthma, cystic fibrosis, or severe inflammatory conditions, the bronchi contain mucus or secretions. In these cases, US demonstrates anechoic tubular branching structures known as the sonographic fluid bronchogram. (Yang et al. 1992; Kim et al. 2000). The fluid-filled bronchi have imperceptible walls and may contain air bubbles. These features may differentiate fluid bronchograms from pulmonary vessels on conventional US (Fig. 14), but definitive differentiation is made by color Doppler. The vessels seen within the consolidation on color or power Doppler can be identified as normal pulmonary vessels by their characteristic polyphasic (mainly quadriphasic) pattern depicted with pulsed Doppler (Fig. 15).
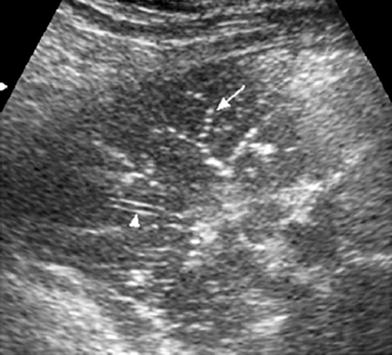
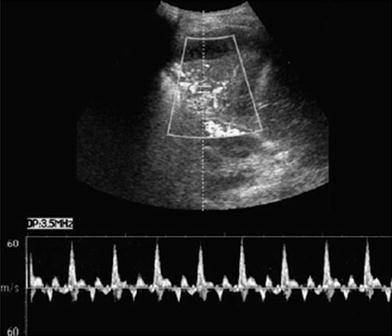

Fig. 14
Sonographic fluid bronchogram in a 6-year-old boy with asthma. Transverse intercostal scan shows bright dots that moved in real time over a hypoechoic background (arrows). The well-defined walls of the pulmonary vessel are clearly seen (arrowhead) while the bronchus wall is imperceptible

Fig. 15
Characteristic spectral Doppler in pulmonary consolidation. Color Doppler US in a patient with pulmonary consolidation shows the characteristic quadriphasic pattern of the pulmonary arteries
Sonographic air bronchograms, fluid bronchograms, air alveolograms, and pulmonary vessels within the lesion are characteristic features of lung consolidation and are never seen in pleural effusion or tumors. Hence, these findings are essential for differentiating between these entities and should be actively investigated (Fig. 16). In peripheral lung consolidation, visualization of pulmonary vessels may be the only sonographic clue to the diagnosis.
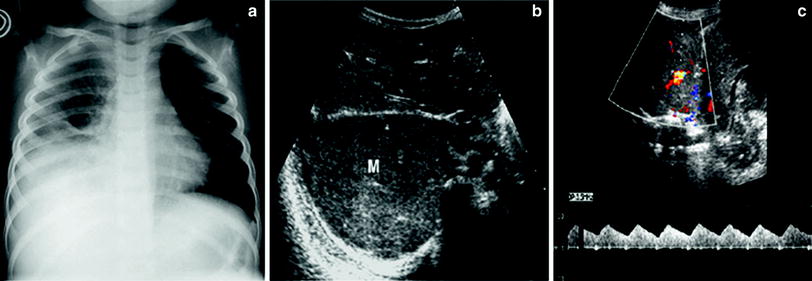

Fig. 16
Extrapleural mass simulating pulmonary consolidation on the chest radiograph a Chest plain film of a 6-year-old boy presenting with fever who has been treated with antibiotics for a week shows opacification of the right lung base compatible with pleuro-pneumonia b Subcostal transverse US view shows a heterogeneous solid lesion (M). No sonobronchograms were identified c The lesions have numerous internal vessels with a systemic wave pattern, a finding that rules out the diagnosis of pulmonary consolidation. The final diagnosis was Yolk sac tumor
According to the degree of vascularization, lobar pneumonia can be classified into three types:
(1)
Well-vascularized pneumonia. In these cases, the consolidation presents a homogenous appearance (similar to the echogenicity of the liver parenchyma) with multiple vascular structures (Fig. 17).
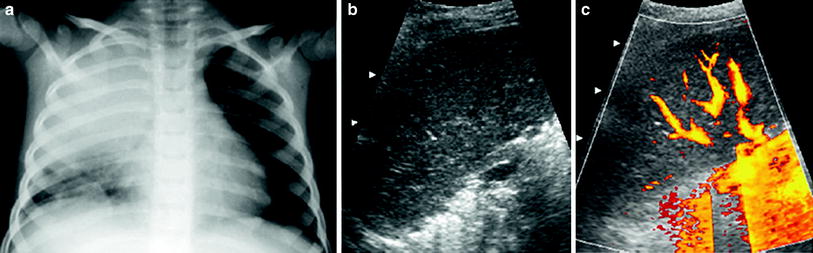

Fig. 17
An 8-year-old boy with well-vascularized lobar pneumonia a Chest radiograph shows opacification of the right upper lobe b The consolidated lung has a homogeneous echogenicity (similar to that of the liver) in this intercostal oblique US scan obtained with the patient in a prone position c Numerous pulmonary vessels are seen on power Doppler
(2)
Poorly vascularized pneumonia without necrotic areas. In contrast to the first type, the number of vessels in the consolidated lung is scant, but the lesion remains homogenous (Fig. 18).
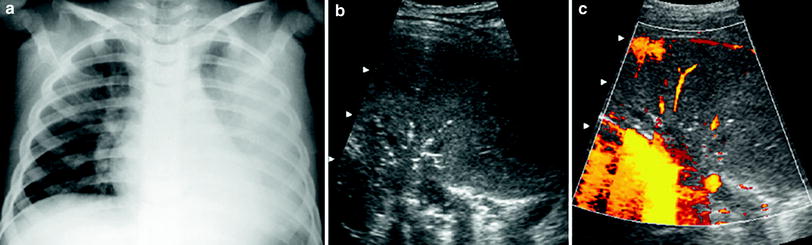

Fig. 18
A 6-year-old girl with poorly-vascularized pneumonia a The chest radiograph discloses evidence of left lung opacification b Intercostal oblique US scan with the patient in a prone position shows homogeneous appearance of the affected lung base with central air sonobronchograms c Very few vessels are seen within the consolidation
(3)
Poorly-vascularized pneumonia with necrotic areas. Very few vessels are visible on color Doppler. Consolidation is usually heterogenous with peripheral areas of cavitation seen as hypoechoic areas, sometimes containing internal echogenic debris (Fig. 19). The absence of vascularization can be localized or involve the whole area of consolidation. (Fig. 20).
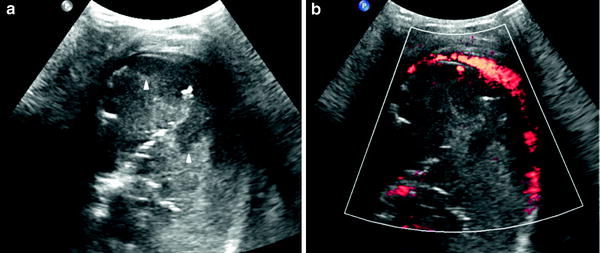
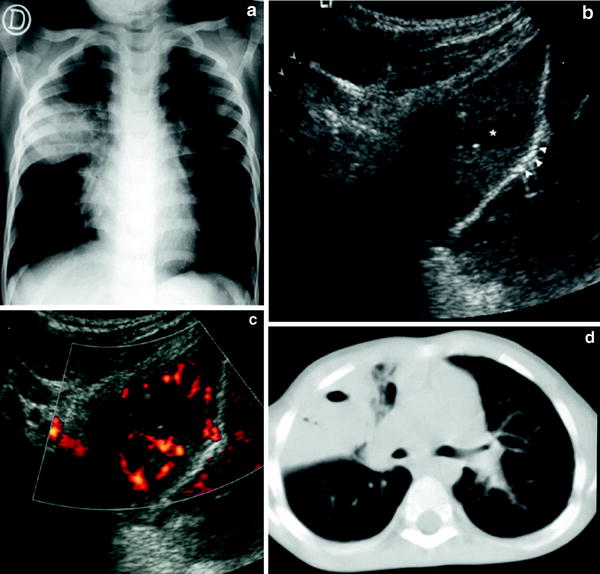

Fig. 19
Pneumonia with necrotic areas in a 5-year-old boy. a Gray-scale US scan shows several hypoechoic images corresponding to necrotic areas within the consolidation (arrowheads). b On power Doppler, no vessel signals are seen in the consolidation, although the pleural hypervascularization is clearly depicted

Fig. 20
Pneumonia with central area of necrosis a The chest radiograph shows a round pneumonia in the anterior segment of the upper right lobe bulging the minor fissure b US study shows a central hypoechoic area corresponding to necrosis (*). The bulged minor fissure is well seen (arrowheads) c Power Doppler shows absence of vascularization in the necrotic area d The necrotic area within the consolidation was confirmed by CT
The third type, known as necrotizing pneumonia, results from necrosis of the lung parenchyma due to occlusion of alveolar capillaries following severe lung infection. Streptococcus pneumoniae is one of the most common microorganisms causing this complication in children (Kerem et al. 1994; Hedlund et al. 1999). In adults, the outcome of necrotizing pneumonia is generally poor and early surgical excision of the gangrenous lung is indicated. However, children can recover completely with medical treatment, although their clinical evolution is long and may require extended hospitalization (Ben-Ami et al. 1993). Thus, in our experience, US provides diagnostic and prognostic information that may influence therapy in children with lobar pneumonia. Contrast-enhanced chest CT in this group of children can provide information similar to that obtained with chest US (Donnelly and Klosterman 1997).
Follow-up studies with US are recommended in children with associated pleural fluid, those with lobar pneumonia and severe clinical symptoms, and those who fail to respond to antibiotic therapy.
When studying consolidation, the radiologist should be aware of the inability of US to determine the extent of a deeply affected area. Acoustic reverberation artifacts, caused by areas of aerated lung interposed between the transducer and the deep area of interest, can hinder visualization of the entire consolidation.
3.5 Interstitial Disease
Several authors have described the value of US for assessing interstitial lung lesions (Agricola et al. 2005; Lichtenstein et al. 2009; Coley 2011). The findings obtained are based on the interaction of the sound beam with thickened subpleural interlobular septa, which produces the so-called B lines (vertically oriented echogenic lines). Some researchers have classified B lines as: B7 lines (7 mm apart), which indicate thickened interlobular septa, and B3 lines (3 mm apart), considered the equivalent of the ground-glass appearance on CT (Stefanidis et al. 2011).
The US patterns seen in several lung diseases affecting premature and newborn infants have been reported (Copetti and Cattarossi 2007; Lovrenski 2012). In premature infants, respiratory distress syndrome (RDS), the clinical expression of surfactant deficiency and generalized alveolar-interstitial syndrome, shows a characteristic US pattern of bilateral, diffuse, compact B lines (Avni et al. 1990, 1996) (Fig. 21). Patients with this condition are treated with surfactant administration, and US is of value for following up the response to therapy (Cattarossi et al. 2010). It is known that surfactant administration rapidly improves pulmonary aeration, but the initial US appearance of the lung can persist even several days after treatment, indicating that lung fluid does not resolve as fast as pulmonary aeration (Copetti et al. 2008).
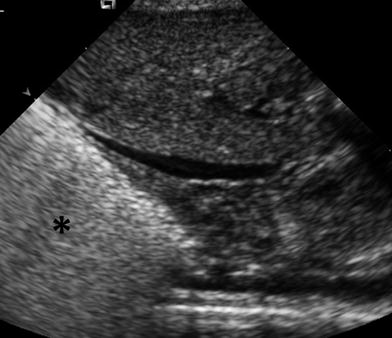

Fig. 21
Characteristic US appearance of hyaline membrane disease. Longitudinal scan shows uniform hyperechogenicity of the right lung base. Compare with normal aerated lung in (Fig. 1)
Numerous B lines, somewhat less compact than in RDS, can be seen in bronchopulmonary dysplasia, a chronic lung disease occurring in premature infants undergoing oxygen therapy (Pieper et al. 2004) (Fig. 22).
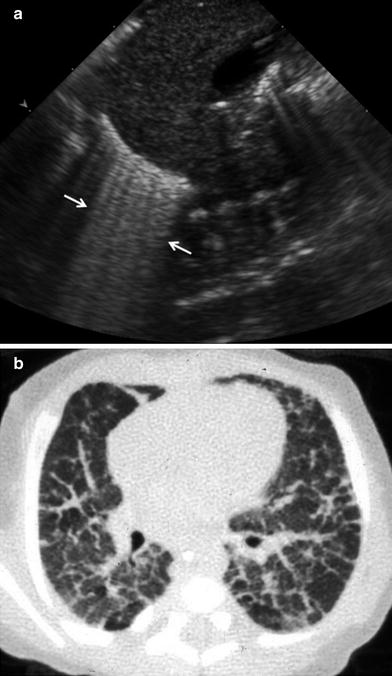

Fig. 22
Premature infant with bronchopulmonary dysplasia. a Longitudinal US scan shows abundant B lines adjacent to the diaphragm. b Corresponding CT scan clearly shows thickening of the interlobular septa, the cause of the B lines on US
Transient tachypnea of the newborn (TNN) usually presents characteristic findings on chest X-ray. Nonetheless, severe cases may show features similar to those seen in pneumonia and sepsis. In patients with TNN, a typical US sign known as the “double lung point” has been reported (Copetti and Cattarossi 2007; Volpicelli 2011b) and provides a clue for the diagnosis. This feature refers to a clear difference between the US pattern seen in the upper and lower lung fields. More compact B lines are seen at the lung bases due to their greater volume.
Knowledge of these US patterns may help neonatologists clarify certain clinical situations without the need for additional plain films and radiation exposure.
3.6 Lung Tumors
Primary lung tumors, including blastoma, mucoepidermoid carcinoma, hemangiopericytoma and rhabdomyosarcoma, are rare in children. The most common of these is pulmonary blastoma. It usually has a complex echogenic appearance and is located in the periphery of the lung. Due to the peripheral location of this lesion, sonography can be used to guide percutaneous biopsy of the mass.
4 Pleural Space
4.1 Normal Appearance and Artifacts
The pleura is a very superficial structure that is well visualized by US. It is composed of two membranes, the visceral and parietal pleurae, separated by a potential space seen as a thin hypoechoic band during respiration in real time. On intercostal longitudinal scans, the pleura is visualized as a highly echogenic linear structure that acquires a curving configuration in the transverse view (Fig. 23). The sonographist should be aware of certain features related to the pleura that are of great diagnostic value. In real-time sonography, the visceral pleura moves with the respiratory excursions, and recognition of this movement provides clues to the diagnosis of several conditions, such as pneumothorax and pleural infiltration by pulmonary or extrapleural tumors. The absence of visceral pleura movement is indicative of pneumothorax. Similarly, the absence of pulmonary tumor movement during respiration indicates that the pleura is infiltrated (Wernecke 2000).
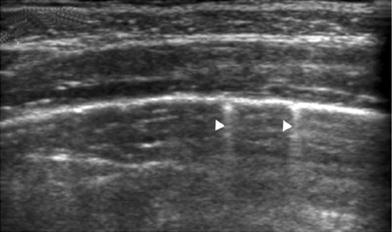

Fig. 23
Intercostal transverse view of the normal pleura. The curving pleuralung interface is well visualized. The small echogenic dots superimposed on the linear interface (arrowheads) represent air in the alveoli as they glide against the pleura with respiratory motion
The pleural surface is a strongly reflective structure, and when the US beam strikes it at certain angles, mirror artifacts (apparent duplication of adjacent structures) can occur. For example, intercostal muscles of the chest wall can reflect off the pleura and be projected over the lung, simulating pulmonary disease.
4.2 Pleural Effusions
Sonography is considered highly sensitive for confirming suspected pleural effusion on AP chest plain films and is often used for this purpose (Eibenberger et al. 1991; Kocijancic et al. 2003). We believe sonography should replace the routine practice of lateral decubitus plain film confirmation in these patients.
On US imaging through an intercostal approach, pleural fluid is identified as a band-like collection separating the parietal and visceral pleura surfaces. When scanning through the abdomen, the collection is seen just above the diaphragm, blurring the costophrenic angle (Fig. 24). Several sonographic signs typical of pleural fluid help to distinguish pleural effusion from ascites. The three most important are the presence of septa within the collection that move with respiration, the “crus sign,” and the “bare area sign” (Seibert et al. 1998). The crus sign, which is pathognomonic of pleural collection, results from displacement of the diaphragmatic crus away from the spine due to interposition of fluid between this structure and the vertebral column (Fig. 25). The “bare area” refers to the posterior part of the right lobe of the liver, which is directly attached to the diaphragm without the covering layer of peritoneum. Peritoneal fluid cannot extend behind the right lobe at this point; thus, all fluid collections visualized behind the bare area are necessarily located in the pleural space.
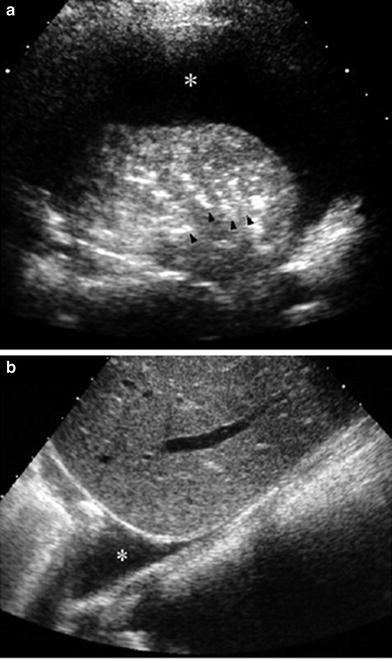
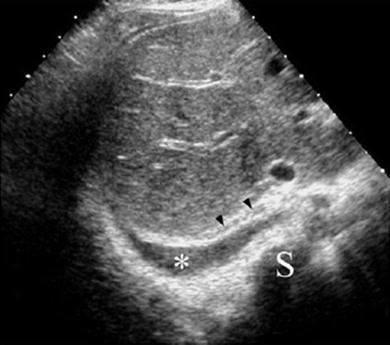

Fig. 24
Sonographic appearance of pleural effusion a The pleural fluid (*) is seen as an anechoic band between the parietal and visceral pleurae in this intercostal, transverse US scan. Note air-bronchograms (arrowheads) in the consolidated lung b Longitudinal sector scan through the liver shows the pleural fluid (*) blurring the costophrenic angle

Fig. 25
Sector transverse US scan through the liver shows useful sonographic findings that differentiate pleural effusion from ascites. Pleural effusion (*) displaces the right diaphragmatic crus (arrowheads) away from the spine (S) and extends behind the right posterior portion of the liver (bare area)
One valuable advantage of sonographic study of pleural effusion is that it enables characterization of the nature of the fluid (Yang et al. 1992; Calder and Owens 2009). Simple effusions are anechoic, whereas complicated effusions present one or more of the following features: weakly echogenic debris with a swirling movement in real time (Fig. 26), mobile fibrin strands, septations (Fig. 27), and a honeycomb appearance (Fig. 28). Recognition of the septated nature of the pleural collection, information that influences patient management, is not usually provided by chest CT scans (Fig. 29) (Kurian et al. 2009).
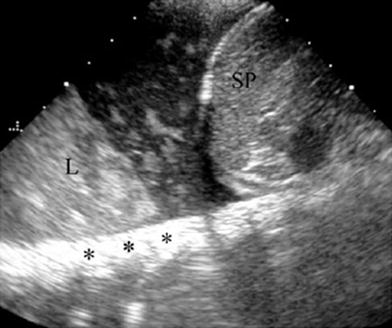
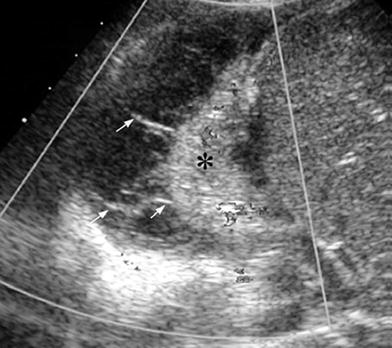
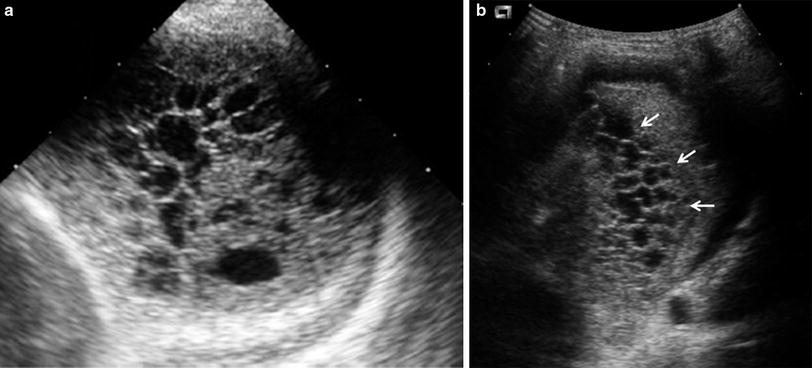
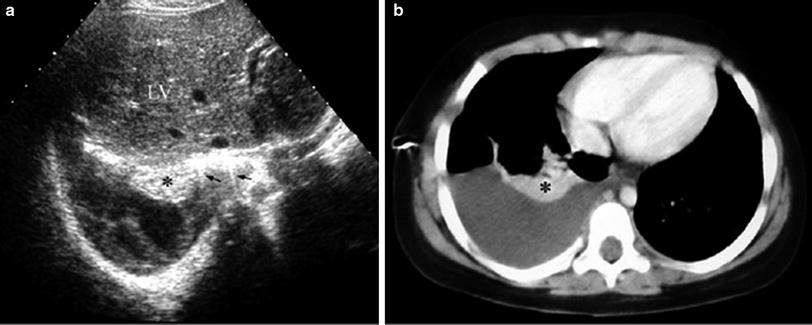

Fig. 26
Pleural effusion with floating debris. Longitudinal transdiaphragmatic US demonstrates pleural effusion containing echogenic particles (evidencing its exudative nature) located between the spleen (SP) and the consolidated left lower lobe (L). The high echogenicity of the ribs (*) results from good transmission of the sound beam through the consolidated lung and pleural fluid

Fig. 27
Right sector intercostal longitudinal view demonstrates a collapsed right lower lobe (*) surrounded by pleural effusion with multiple fibrin bands (arrows)

Fig. 28
8-year-old boy with streptococcal pneumonia. a In this left transverse intercostal scan, the pleural space is filled with profuse septations producing a honeycomb appearance. b Transverse US scan in a different patient shows a similar honeycomb pattern in the pleural space along the left lung fissure (arrows)

Fig. 29
Transverse US scan (a) through the liver (LV) and enhanced CT scan (b) of a 5-year-old girl with pleural pneumonia. The compressed, atelectatic lung (*) is clearly seen in both studies, but the septated nature of the pleural collection is only evident on the US scan. Note comet tail artifacts in the normal aerated lung adjacent to the collapse (arrows)
The sonographic appearance of pleural effusion can be related to the classical division of pleural fluid into exudate or transudate, which depends on its protein content, pleural: serum LDH ratio, and other biochemical parameters. On sonography, both transudates and exudates can be anechoic, but collections presenting some of the complicated features mentioned above are always exudates.
Most exudative pleural effusions in pediatric patients are of infectious origin (Alkirinawi and Chernick 1996). These collections are known as parapneumonic effusion or empyema. The diagnosis of empyema is established when pleural fluid is grossly purulent, organisms are identified on gram stain or culture, pleural fluid white blood cell count is greater than 5 × 109 cells/L, pH is below 7.0, or glucose level is less than 40 mg/dL.
There is still a great deal of controversy around the clinical management of parapneumonic effusion and empyema. (Feola et al. 2003; Wells and Havens 2003; Hogan and Coley 2008; Calder and Owens 2009). Two main treatment approaches are used: nonoperative, in which patients are treated with antibiotics alone or combined with chest drainage and fibrinolytic instillation, and operative, consisting of pleural debridement or decortication. The goal of both these treatment methods is to evacuate infected debris and re-expand the lung, and to reduce hospital stay and morbidity.
The presence of a honeycomb pattern on US is not a contraindication for pleural drainage and fibrinolytic therapy (Wells and Havens 2003). Surgery should be used only in patients who do not respond to this therapy.
In our experience, small exudates can rapidly (within 24 h) increase in volume and change their sonographic appearance, despite antibiotic therapy. Thus, close sonographic monitoring of children with empyema is recommended. In addition, US is a valuable tool for localizing loculations for thoracocentesis or thoracostomy tube placement (Coley 2002). The incidence of pneumothorax is considerably reduced when pleural taps are sonographically guided, particularly in the case of small or loculated collections (Raptopoulos et al. 1991; Shankar et al. 2000).
4.3 Opaque Hemithorax
Opaque hemithorax on chest films is one of the main indications for chest US. In most cases, opaque hemithorax is caused by massive pleural effusion, but before pleural puncture is considered, US should be performed to confirm that the opacity is actually caused by fluid accumulation and not by a solid lesion or a mixture of both components (Fig. 30).
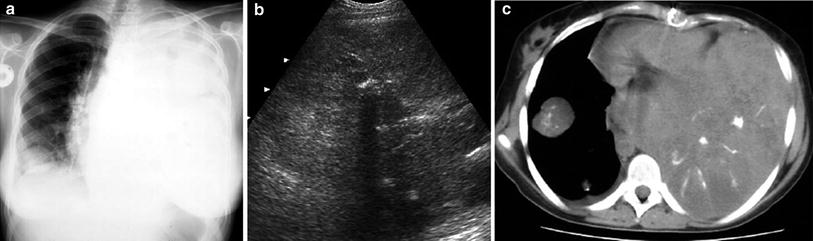

Fig. 30
Opaque left hemithorax in a 17-year-old boy with osteogenic sarcoma of the left femur a The chest radiograph shows opaque hemithorax with increased volume, suggesting massive pleural effusion, as well as a nodule in the right lower lobe b Intercostal transverse US scan rules out pleural fluid and demonstrates that the left hemithorax is occupied by a huge solid mass with echogenic areas and acoustic shadowing, suggestive of calcifications c Unenhanced CT demonstrates a huge partially ossified metastasis on the left and the right pulmonary nodule
4.4 Pleural Tumors
Primary tumors originating in the pleura, such as mesothelioma, are very rare in children, and neoplastic involvement of this structure is most often due to metastasis. Metastatic disease to the pleura often causes large pleural effusions that are probably due to impaired lymphatic drainage. This secondary pleural fluid, which can be profuse in some patients, may mask the tumor mass on chest X-rays, and in these cases, ultrasound is particularly helpful (Fig. 31). Metastatic involvement of the pleura can be caused by various intrathoracic or extrathoracic tumors, such as Wilms tumor, lymphoma, neuroblastoma, and rhabdomyosarcoma (Fig. 32).
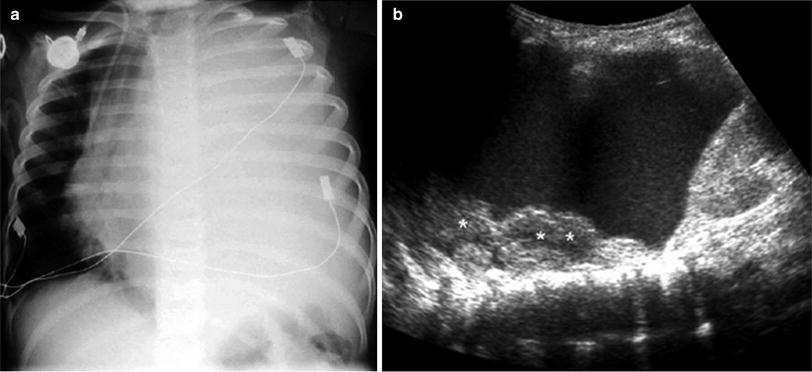
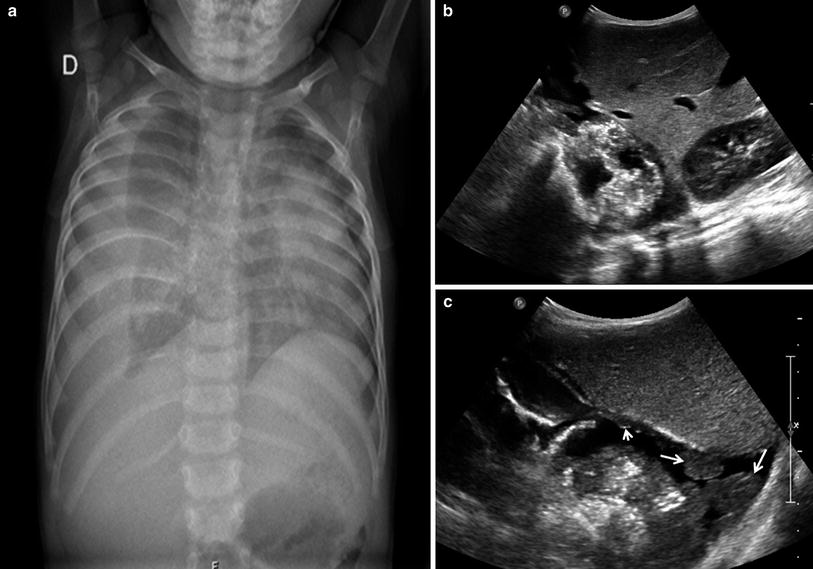

Fig. 31
Massive pleural effusion in a patient with lymphoma a Chest radiograph shows opaque left hemithorax with mediastinal displacement to the right b Several pleural-based lymphomatous nodules (*) are identified through the pleural fluid on US examination

Fig. 32
Thoracic neuroblastoma with pleural implants in a 2-year-old boy. a Chest X-ray shows pleural effusion, mediastinal displacement, and increased intercostal spaces and inconclusive calcifications on the right side. b Longitudinal US scan shows a huge mass with abundant microcalcifications that were not conclusively identified on chest X-ray. c US scan at a different level depicts multiple masses of different sizes, corresponding to pleural metastasis (arrows)
4.5 Pneumothorax
Chest X-ray remains the method of choice for the diagnosis of pneumothorax, but several projections may be required to reveal its presence. In severely ill patients or trauma patients who cannot be easily moved, pneumothorax can be confirmed or ruled out by US without changing the patient’s position (Barillari and Kiuru 2010; Volpicelli 2011a).
Small pockets of air in the pleural space appear as bright, echogenic lines or points. In contrast to what is seen in the normal aerated lung, the image of air in the pleural space does not show comet tail artifacts (Fig. 33). A large pneumothorax can impede visualization of visceral pleura movement, an important indirect sign of air in the pleural space. The presence of air and fluid in the pleural space (hydropneumothorax) results in an air-fluid level on US that can produce a particular movement in real time known as the “curtain sign” (Ben-Ami et al. 1993; Lichtenstein et al. 2000).
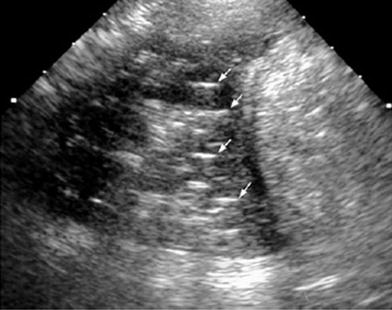

Fig. 33
Longitudinal transdiaphragmatic US scan of the lung base in a 7-year-old girl. Air bubbles within the pleural effusion are seen as linear echogenic images without comet tail artifacts (arrows)
5 Chest Wall
The chest wall provides a structural framework that protects the intrathoracic organs. It is composed of nerve, bone, muscle, and vasculature, which can be affected by congenital malformations, infections, tumors, and metabolic diseases. Ultrasound is often the first test requested by clinicians to investigate chest wall abnormalities and disease; hence, the radiologist should be familiar with the characteristic US findings of these conditions.
Normal variants of the anterior chest wall related to the sternum, ribs, and costal cartilage may produce an asymptomatic palpable lump. These anomalies have been well described on CT studies (Donnelly and Frush 1999), but some of them, particularly those related to asymmetrical costal cartilage, can be easily recognized on US (Fig. 34).
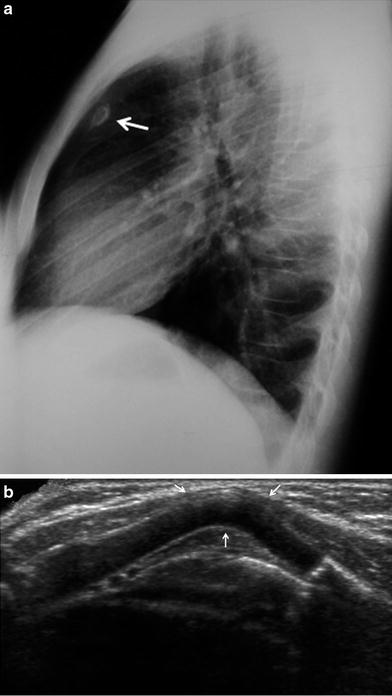

Fig. 34
Fourteen-year-old girl with a palpable lump in the left upper quadrant of the chest. a Lateral plain film shows a small nodular image with a dense peripheral ring (arrow) behind the sternum. b Transverse US view confirms sharp angulation of a rib cartilage as the cause of the lump
Lymphatic and vascular malformations, and hemangiomas are common soft tissue masses occurring in the chest wall. Lymphatic malformations are usually visualized as single or multiple cystic images that may increase in size due to internal hemorrhage. In addition to establishing the diagnosis, US can be used to guide percutaneous sclerosing therapy for this condition. Vascular malformations are suspected clinically by skin discoloration (reddish or bluish, depending on the depth of the lesion), and the US findings vary according to the type (arterial, venous, or capillary) (Dubois et al. 2002). Hemangioma is seen as a well-circumscribed mass, superficial to the intercostal musculature (Fig. 35). Doppler study reveals a pattern of high systolic and diastolic shifts. Lipomas and neurofibromas are other benign soft tissue masses encountered in children (Siegel 2002; Smeets et al. 1990).
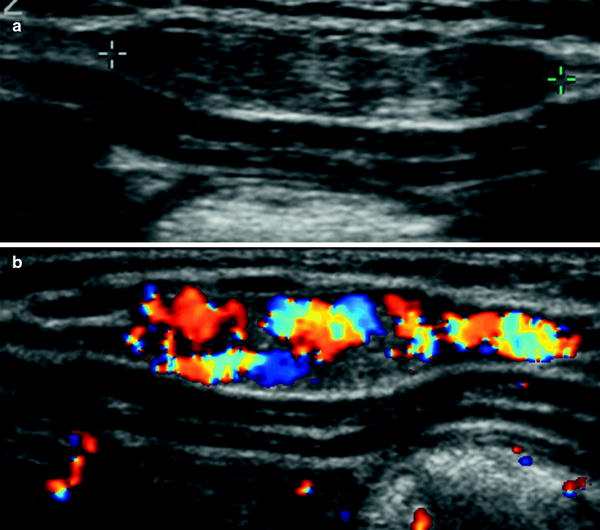

Fig. 35




Chest wall hemangioma in a 1-month-old infant. a Gray-scale US demonstrates a solid elliptical mass superficial to the intercostal musculature (arrow). b On color Doppler, the mass is seen to have prominent vascularization
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree