Fig. 13.1
One of the many illustrations of the human retina done by SR Cajal around 1880 that earned him the Nobel Prize in Medicine. While some details are incorrect, he did recognize the vertical orientation of the layers and their synaptic alignment (Courtesy of Instituto Cajal (CSIC) Madrid, Spain. Cajal Legacy)
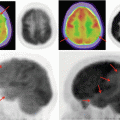
As with CT and MRI, OCT unveiled a previously concealed, anatomical region protected from the in vivo observations. As the reproducibility and reliability of OCT improved, clinical trials were a natural added dimension to its portfolio (Fig. 13.2a–c). Biomedical engineering achievements have transformed OCT from time-domain (TD) imaging, adept at capturing abnormalities of the vitreo-retinal interface, to spectral-domain (SD) technology that has defined in vivo imaging of the structure of the photoreceptors of the outer retina (Fig. 13.2a–c).
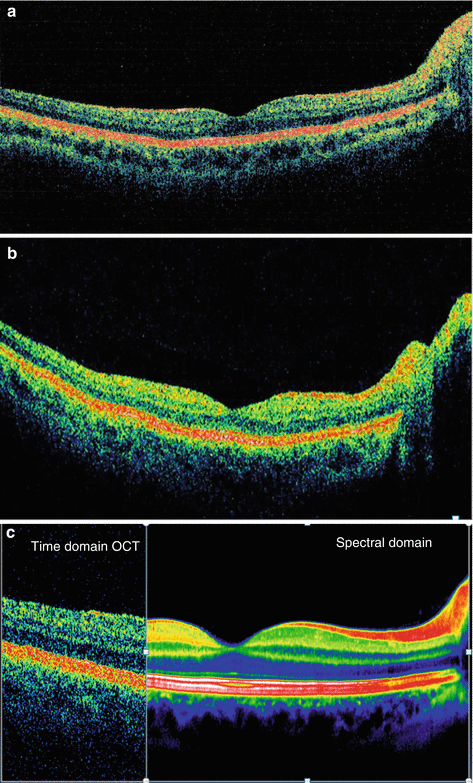

Fig. 13.2
(a) Time-domain OCT. (b) Spectral-domain scan. (c) Time domain versus spectral domain (c Courtesy of Heidelberg Engineering)
TD-OCT permitted only 2-dimensional imaging; however, SD-OCT, also referred to as Fourier-domain OCT, greatly improved data-acquisition speeds facilitating 3-dimensional imaging.
In addition, OCT is especially enticing for both clinical use and regulatory trials since the testing paradigm encompasses two perfect medical triads: one for the patient and one for clinical trial specialists and regulators. The patient triad includes its painless, non-contact, noninvasive nature. Light is the only imaging modality required and oral or intravenous contrast agents are not necessary. No complications of OCT have ever been reported. Clinical trial professionals profit in both the protocol and regulatory arenas by the remarkable correlation of qualitative findings with the in vivo histology of the retina and optic nerve as well as the highly refined reproducibility and reliability of the quantitative measurements.
OCT is not a replacement for fundus photography but rather incorporates an added dimension of visualizing living optic nerve and retina again and again without damage to the anatomy or physiology of these structures. Previously sequestered secrets and signatures of both ophthalmic and neurologic diseases are now lucid and transparent.
Therefore, in this volume dedicated to clinical trials, we applaud a new technology whose birth came through ophthalmology but whose offspring will foster major advances in every medical discipline in which we have access to in vivo tissue through endoscopes, catheters, or intraoperative probes. The initial lessons learned in ophthalmology have already found applications in neurology which will share the dais with ophthalmic diseases in this chapter. We, however, confidently predict that the next edition will embrace OCT as a clinical trial biomarker in gastroenterology, neurosurgery, cardiology, and pulmonary medicine. Through the collaborative efforts of basic researchers, innovative clinicians (not “providers”), and medical device makers, OCT has improved the care and quality of life of patients not only with potentially blinding ophthalmic diseases but also with potentially disabling and life-threatening conditions in the central nervous system.
Basic Technology
OCT utilizes the principles of interferometry and coherent light to produce images of both diagnostic and regulatory quality. However, the realm of OCT did not start with medical imaging elegance. The first commercial use of OCT was not in medicine but in the world of art history and old master painters to determine if multiple paintings were layered on a single canvas. Due to the expense of proper canvases, many Old World artists were inclined to paint over previous paintings, entombing the original work. On many occasions, the artwork concealed beneath the surface of the visible image was much more valuable and more important than the surface facade. In a similar way, what lays beneath the surface of the retina and optic nerve are often more important than what is seen with the ophthalmoscope.
Before we explain how OCT has achieved its current sophistication, we will review the fundamental technology. When a wavelength of light encounters the boundary of two different media, the light may be reflected, refracted, and/or absorbed. The first step for OCT is the acquisition of the reflected light by a capture device.
Next, the algorithm of interferometry, an electromagnetic principle, is used. “Interference” can be either constructive or destructive for imaging purposes depending upon the relative phases of the waves. OCT employs the interaction of waves coherent with each other to form images by sectioning or “tomography,” a physical application used in CT, MRI, and ultrasonography.
One of the limiting technological issues for the first OCT instruments was the number of A scans that could be performed in a finite time, thereby limiting image resolution (Fig. 13.3). Improvement in this engineering domain has increased the number of A scans and shortened the capture time for this biomechanical variable.
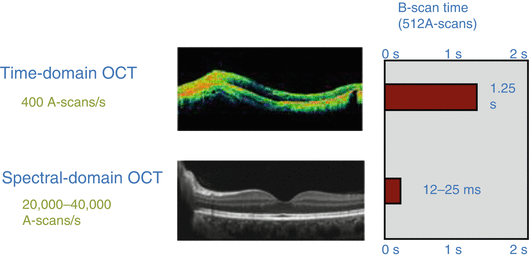

Fig. 13.3
Spectral-domain OCT greatly increased the number of A scans performed per second. That advance as well as improved lasers and minimizing movement artifacts increased both the imaging quality and coefficients of variability of OCT qualitative and quantitative analysis (Courtesy of Heidelberg Engineering)
Another similarity with MRI, specifically orbital imaging, demanded correction for normal, continuous, physiological movements of the eyes in the resting stage, termed microsaccades. Until MRI software conquered this obstacle, orbital MRI scans were blurred and substandard due to movement artifacts. By using a variety of techniques to insure image stabilization and registration, SD-OCT devices have reduced this source of artifact (Fig. 13.4) and hence further improved image quality and decreased the coefficient of variability for measuring the dimensions of the retina.
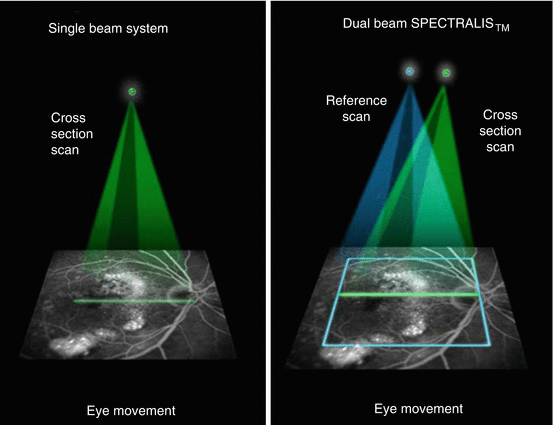

Fig. 13.4
With the single beam system used in TD-OCT and some SD-OCT devices (left picture), the eye location is not known unless a second reference scan is used (Courtesy of Heidelberg Engineering), as shown on the right picture
Because OCT requires light to be precisely focused upon the retina, imaging is often limited by the dimensions of the eye. Somewhat smaller than normal eyes, hyperopia (far sightedness) greater than 6 diopters, and somewhat larger eyes, myopia (near sightedness) greater than 6 diopters, may induce errors in image resolution and the measurement algorithms of the peripapillary retinal nerve fiber layer thickness and the macular thickness.
What Is Normal?
When TD-OCT was introduced into the commercial market, the technology was patent protected. As SD-OCT emerged, patent protection was no longer possible because Fourier analysis was required to improve image quality. Fourier analysis is considered to be common scientific knowledge, not subject to patent enforcement. SD-OCT facilitated competition among device manufacturers in the commercial market, which has produced improved technology and helped to control price. The hardware and software platforms from various manufacturers differ fundamentally in methodology of image processing as well as the definition of the posterior border of the retina. Does the retina end at the inner border of the retinal pigment epithelium (RPE), the outer border of the RPE, or Bruch’s membrane? The answer to that question depends upon which device a clinician or clinical trial selects (Tables 13.1 and 13.2). The most important conclusion from the different definitions of the borders of the retina becomes the inability to transfer and merge qualitative data from one OCT platform to another for determining baseline measurements as well as changes over time during a clinical trial. Measuring percentage change over time may be a way to approach this conundrum but that is somewhat flawed since the basic dimensions are different, and this approach has not been accepted by regulatory authorities. Tables 13.1 and 13.2 further illustrate this problem, which becomes very significant for international trials where use of a single device is rarely possible unless supplied by the sponsor. Ultimately, regulators may declare which platforms and measurements algorithms are acceptable for specific trials.
Table 13.1
Summary of specifications of the five commercial OCT instruments
OCT instrument | Axial resolution (μm) | A-scan speed (scans/s) | Macular thickness outer boundarya | Manufacturer | Software version, software protocol |
|---|---|---|---|---|---|
Stratus | 8–10 | 400–600 | IS-OS junction | Carl Zeiss Meditec, Inc., Dublin, CA | v4.0; macular thickness map protocol |
3D OCT-1000 | 5–6 | 25,000 | Inner RPE | Topcon, Inc., Paramus, NJ | v2.12; 3D macular protocol |
Optovue (RTVue-100) | 5–6 | 26,000 | Outer RPE | Optovue, Inc., Fremont, CA | v3.5; (E)MM5 and MM6 |
Cirrus | 5 | 27,000 | Outer RPE | Carl Zeiss Meditec, Inc., Dublin, CA | v3.0; macular cube 512 × 128 |
Spectralis | 4–6 | 40,000 | Bruch’s membrane | Heidelberg Engineering, Inc., Heidelberg, Germany | v3.2; macular volume protocol |
Table 13.2
Average central macular thickness (microns) ± standard deviation across five OCT devices in normal and pathologic eyes
OCT device | Normal macula | Pathologic macula |
|---|---|---|
Stratus | 185.3 ± 16.7 | 256.2 ± 166.4 |
Cirrus | 249.1 ± 26.5
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|