Fig. 1
Historic view on early and late mucosal effects related to radiotherapy (with permissions from Rubin and Casarett 1968)
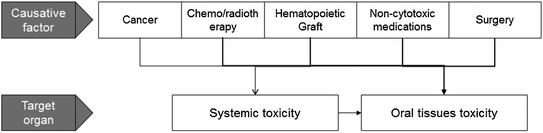
Fig. 2
Oral toxicities may be resulted from the cancer, treatment for the cancer, pharmacologic treatment for cancer complications or from systemic side-effects
Treatment for malignant disease produces unavoidable toxicities to normal cells. The oral tissues are highly susceptible to direct and indirect toxic effects of cancer chemotherapy and ionizing radiation. Some of the oral complications in cancer patients are related directly to the effects of chemo-radiotherapy on oral tissues and others are the indirect consequence of systemic toxicities (Fig. 2). Patients undergoing cancer treatment may develop oral complications associated with their modified immune status post hematopoietic stem cell transplantation (HSCT) or with the supportive care (e.g., bisphosphonate-related osteonecrosis). Surgical tumor removal may also cause oral and nutritional problems.
The tissue response depends on factors related to the form of cancer treatment and the patient. Treatment-related factors include: treatment modality (radiotherapy, chemotherapy, surgery, combination protocols, hematopoietic stem cell transplantation [HSCT]); the level of exposure to cytotoxic agents (e.g., intensity of protocol, field of radiation); and use of preventive measures. The patient-related factors include age, genetics, underlying malignant disease, immune status, co-morbidities, concurrent medications, nutritional status, history of previous cancer treatments, and compliance. Interaction of these elements governs the risk, course and severity of tissue injury.
With the emergence of new cancer treatments, the response is even more variable. For example, the introduction of reduced-intensity HSCT changed the profile of oral complications compared to those following myeloablative HSCT. Similarly, more advanced radiation technique may minimize some of the characteristic oral complications. Lastly, the introduction of targeted-therapy in oncology has caused a new series of oral complications.
Whereas the traditional bio-continuum of adverse effects (Fig. 1) was a linear progression from early acute oral complications to late chronic oral complications, the current understanding of the dynamics of the development of oral complications is multi-directional. Late complications may have an acute nature and vice versa. Tissue injury may be transient or permanent. The temporal behavior of each oral complication requires an appropriate clinical approach and patient education.
The late adverse oral effects, irrespective of their cause, are associated with loss of important oral functions such as eating, tasting, drinking, swallowing and speaking. Patients may also suffer dehydration, dysgeusia, ageusia, malnutrition and weight loss (Epstein et al. 2001, 2002). The patient’s quality of life (QOL) can be severely affected, impacting their daily lives (Talmi 2002). When there are esthetic considerations such as mutilation or malformation, the impact on the psychological state of the patient can be profound (McMillan et al. 2004; Nordgren et al. 2008).
Details of the late oral complications in cancer patients are presented in this chapter, including their pathophysiology, clinical presentation, and potential treatment. According to the template of this textbook, a summary of the normal anatomy and physiology will provide the basis for understanding the pathology.
2 Anatomy and Histology
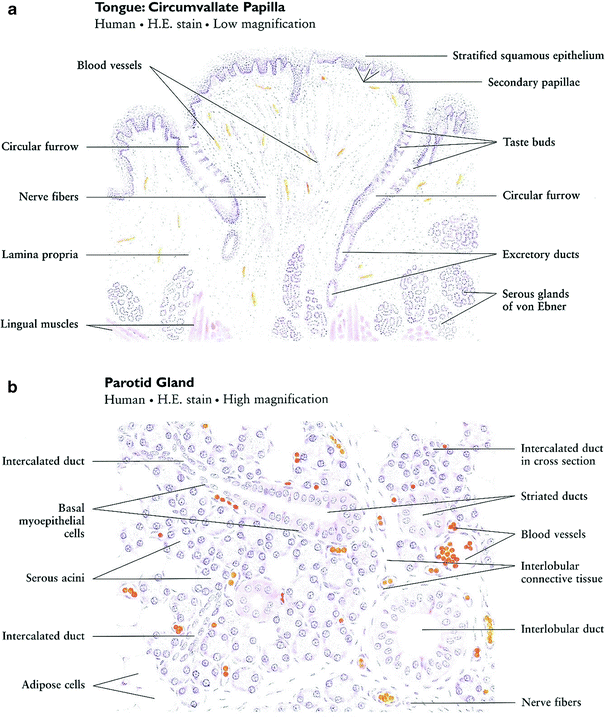
The oral cavity originates with the lips and extends posteriorly to the oropharynx (Fig. 3). It can be diagrammatically sketched as an elongated box with the cheeks contributing the side walls, the palate contributes the ceiling, and the floor of the mouth contributes the base. The supporting-frame for this three-dimensional box is composed of the dental arches positioned on the upper and lower jaw bones. Within this space lies the tongue. The oral mucosa covers the interior of the oral cavity, including the tongue, and merges into the gingival tissue covering the alveolar jaw bones. The major and minor salivary glands, the muscles, the blood vessels, the nerves, the lymphatic system and the taste buds are within the walls of the theoretic box structure of the oral cavity (Fig. 3).
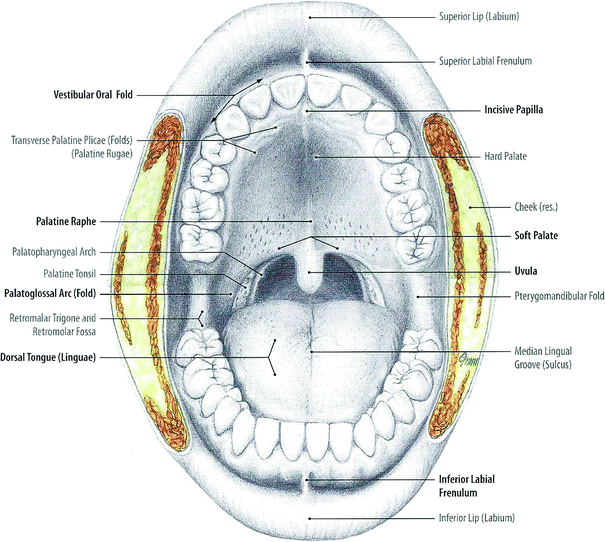

Fig. 3
Oral cavity: front view (With permission from Tillman and Elbermani 2007)
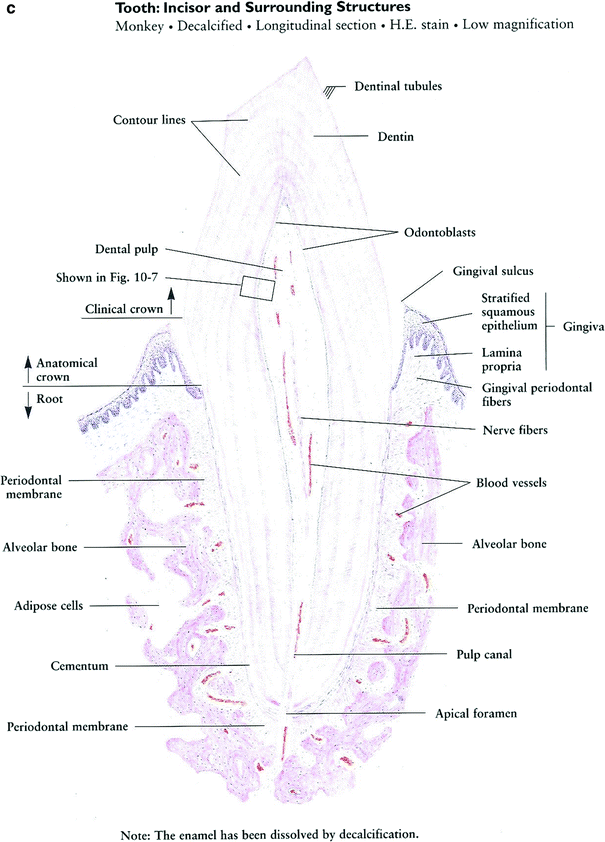
Although the oral mucosa serves as a continuous coverage, the characteristic of the oral mucosal are not uniform through the oral cavity and are adjusted to the specific function of each oral surface. The mucous membranes of the oral cavity and pharynx are characterized as masticatory, lining, or specialized. The masticatory mucosa is bound down tightly by the lamina propria to the underlying bone. It covers the hard palate and gingiva and is characterized by keratinized epithelium that provides resistance to friction during mastication. The lining mucosa is characterized by non-keratinized epithelium, which provides flexibility and mobility that is needed during oral function. The specialized oral mucosa is found on the dorsum of the tongue and contains various types of papillae and taste buds (Fig. 4a).
The mucosal epithelium rests on a basement membrane. Below this, minor salivary glands and numerous small arteries, veins, and lymphatic vessels are found. Normal mucous membrane is pink to pale pink, depending on the thickness of the mucosal epithelium and amount of blood flowing through the underlying microvasculature.
There are three paired sets of major salivary glands—the parotid, submandibular, and sublingual glands—and hundreds of minor salivary glands scattered in the submucosa throughout the oral cavity, except the gingiva and anterior part of the maxilla. A typical salivary gland is a composite of numerous functional units: secretory end pieces known as acini, collecting ducts, and a framework of myoepithelial cells and connective tissue (Fig. 4b). There are two kinds of acinar cells, mucous and serous. Secretions from each of the acini pass through the intercalated ducts, then the striated (secretory) ducts and terminal lobar (excretory) ducts before traversing the major salivary duct into the oral cavity.
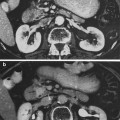
The main body of the tooth is made up of dentin (chemical composition of the calcium-phosphate-hydroxide apatite being 70 % inorganic and 30 % organic). Covering the crown portion of the tooth is the enamel (96 % inorganic, 4 % organic). The root portion is covered by cementum (50 % inorganic, 50 % organic), in which is imbedded protein fibrils that attach the tooth to the alveolar bone that surrounds the socket. The inner portion of the tooth—the pulp—is composed of blood vessels, lymphtics, nerves, and fibrous connective tissue (Fig. 4c). The tooth is embedded in alveolar bone. The compartment around the teeth is termed periodontium and the portion that supports the tooth in bone is the periodontal ligament. With aging and loss of teeth alveolar bone is physiologically resorbed.
The mandibular bone is characterized by cortical bone (lamellar bone and Haversian systems) that covers the spongy or marrow bone. The tooth sockets are lined by this compact bone. The posterior mandible (ramus) extends vertically ending in the condylar process that forms the temporomandibular joint, allowing opening and lateral movements for speech, mastication and expression.
The internal structure of mandibular bone responds to mechanical stresses by remodeling of marrow trabeculae, which are lined by osteoblasts and osteoclasts on the surface, and osteocytes in inner lacunae. The marrow itself contains blood vessels, fat, and fibrous connective tissue.
3 Physiology and Biology
The oral mucosa has an important role in protection as it creates a barrier between the oral environment and the circulation. Its capability to withstand friction allows food chewing and swallowing. Additionally, receptors in the oral mucosa respond to temperature, touch and pain and the tongue has the unique capability of taste detection.
The salivary glands generate saliva which has important physiologic functions. It protects the oral cavity by providing a washing effect. This clearance of bacteria, debri and sugars, is important to prevent infection and the development of dental and periodontal diseases. Saliva lubricates the oral mucosal surfaces, allowing for tissue protection. Its flow, electrolytes composition and buffering effect, helps prevent demineralization of teeth. Saliva proteins, such as lysozyme, lactoferrin, peroxidase, and secretory immunoglobulin A, have an antimicrobial action. Other salivary molecules, such as mucins and agglutinins, aggregate microorganisms and prevent them from adhering to the oral tissues. Additionally, saliva is important for taste perception and for digestion of the food. Lastly, the moistening of the oral cavity allows for speech and breathing.
Both the teeth and jaw bones are essential for mastication, intelligible speech, swallowing, appearance, and expressions.
Within the complex structure of the mouth, the biofilm of saliva and oral flora is an invisible component that is integrated into the normal physiology of this entire organ. The oral flora is characterized by harmonious interaction between hundreds of bacteria and fungi. This co-existence may alter during changes due to an exogenous triggers (e.g., antibiotics) or endogenous change (e.g., immunosuppression). Some of the normal oral micro-organisms may turn into opportunistic micro-pathogens when the harmonious balance between various oral residents is interrupted.
4 Clinical Presentation
4.1 Overview
Previous editions of this book addressed the oral mucosa, the salivary glands and the teeth and mandible (i.e., jaw bone). This clinical approach is based on the LENT SOMA system that categorizes and grades various toxicities (Tables 1, 2, and 3), and does not differentiate between early and late adverse events. As evidence-based data on oral complications of cancer patients expanded, scales were published for specific oral complications. Several global scales are currently used. For example, the National Cancer Institute published an update for the Common Toxicity Criteria for Adverse Events (CTC-AE ver. 4.0) and a new version is expected in the near future. Selected items from the CTC-AE are presented in Table 4.
Table 1
Mucosa LENT SOMA
|
Mucosa—oral and pharyngeal
|
||||
|---|---|---|---|---|
|
Grade 1
|
Grade 2
|
Grade 3
|
Grade 4
|
|
|
Subjective
|
||||
|
Pain
|
Occasional and minimal
|
Intermittent and tolerable
|
Persistent and intense
|
Refractory and excruciating
|
|
Dysphagia
|
Difficulty eating solid food
|
Difficulty eating soft food
|
Can take liquids only
|
Totally unable to swallow
|
|
Taste alteration
|
Occasional, slight
|
Intermittent
|
Persistent
|
|
|
Objective
|
||||
|
Mucosal integrity
|
Patchy atrophy or telangiectasia
|
Diffuse atrophy or telangiectasia, superficial ulcer
|
Deep ulcer no bone or cartilage exposure
|
Deep ulcer with bone or cartilage exposure
|
|
Weight
|
<5 % loss
|
>5–10 % loss
|
>10–15 % loss
|
>15 % loss
|
|
Management
|
||||
|
Pain
|
Occasional non-narcotic
|
Regular non-narcotic
|
Regular narcotic
|
Surgical intervention
|
|
Ulcer
|
Cleanse
|
Antibiotics or oxidants
|
Debridement + other surgical intervention
|
|
|
Dysphagia
|
Lubricants, diet modification
|
Non-narcotic
|
Narcotic
|
PEG tube and/or surgical intervention
|
|
Taste alteration
|
Minor diet changes (non-acidic)
|
Minor diet changes (semi-soft)
|
Major diet changes (soft)
|
Major diet changes (liquid)
|
|
Analytic
|
||||
|
Color photo
|
Assessment of changes in appearance
|
|||
|
Cytology, biopsy, imaging
|
Rule out persistent tumor
|
|||
|
Smear, culture, antifungal trial
|
Rule out candidiasis
|
|||
Table 2
Salivary gland LENT SOMA
|
Salivary gland
|
||||
|---|---|---|---|---|
|
Grade 1
|
Grade 2
|
Grade 3
|
Grade 4
|
|
|
Subjective
|
||||
|
Xerostomia
|
Occasional dryness
|
Partial but persistent dryness
|
Complete dryness, non-debilitating
|
Complete dryness, debilitating
|
|
Objective
|
||||
|
Saliva
|
Normal moisture
|
Scant saliva
|
Absence of moisture, sticky, viscous saliva
|
Absence of moisture, coated mucosa
|
|
Management
|
||||
|
Xerostomia
|
Occasional saliva substitute Sugarless candy or gum, Sialogogues
|
Frequent saliva substitute or water Sugarless candy or gum, Sialogogues
|
Needs saliva substitute or water in order to eat Sugarless candy or gum, Sialogogues
|
|
|
Analytic
|
||||
|
Salivary flow/quantity/stimulation
|
76–95 % of pre-treatment
|
51–75 %of pre-treatment
|
26–50 % of pre-treatment
|
0–25 % of pre-treatment
|
Table 3
Mandiblea LENT SOMA
|
Mandible
|
||||
|---|---|---|---|---|
|
Grade 1
|
Grade 2
|
Grade 3
|
Grade 4
|
|
|
Subjective
|
||||
|
Pain
|
Occasional and minimal
|
Intermittent and tolerable
|
Persistent and intense
|
Refractory and excruciating
|
|
Mastication
|
Difficulty with solids
|
Difficulty with soft foods
|
||
|
Denture use
|
Loose dentures
|
Inability to use dentures
|
||
|
Trismus
|
Noted but unmeasurable
|
Preventing normal eating
|
Difficulty eating
|
Inadequate oral intake
|
|
Objective
|
||||
|
Exposed bone
|
< 2 cm
|
> 2 cm or limited sequestration
|
Fracture
|
|
|
Trismus
|
1–2 cm opening
|
0.5–1 cm opening
|
<0.5 cm opening
|
|
|
Management
|
||||
|
Pain
|
Occasional non-narcotic
|
Regular non-narcotic
|
Regular narcotic
|
Surgical intervention or resection
|
|
Exposed bone
|
Antibiotics
|
Debridement, hbo2
|
Resection
|
|
|
Trismus and mastication
|
Soft diet
|
Liquid diet, Antibiotics, Muscle relaxant meds
|
NG tube, gastrostomy
|
|
|
Analytic
|
||||
|
Mandibular radiograph
|
Questionable changes or none
|
Osteoporosis (radiolucent)
|
Sequestra
|
Fracture
|
|
Osteosclerosis (radiodense)
|
||||
|
Panograph X-rays/CT
|
Assessment of necrosis progression
|
|||
Additional scales are listed in the RTOC website.
In this edition, common oral complications will be presented, including oral mucosal alterations (late-mucosal injury and cGVHD), taste disorders, salivary gland dysfunctions, infections, neuropathy and chronic pain, complications of the dentition and periodontium, trismus and loss of elasticity, osteoradionecrosis and osteonecrosis, developmental abnormalities and secondary malignancies. The association between the various toxicities and therapies is provided in Table 5.
Table 4
Common Toxicity Criteria for Adverse Events (CTC-AE ver. 4.0)
|
Adverse event
|
1
|
2
|
3
|
4
|
5
|
Definition
|
|---|---|---|---|---|---|---|
|
Cheilitis
|
Asymptomatic; clinical or diagnostic observations only; intervention not indicated
|
Moderate symptoms; limiting instrumental ADL
|
Severe symptoms; limiting self care ADL; intervention indicated
|
–
|
–
|
Disorder characterized by inflammation of the lip
|
|
Dental caries
|
One or more dental caries, not involving the root
|
Dental caries involving the root
|
Dental caries resulting in pulpitis or periapical abscess or resulting in tooth loss
|
–
|
–
|
A disorder characterized by the decay of a tooth, in which it becomes softened, discolored and/or porous
|
|
Dry mouth
|
Symptomatic (e.g., dry or thick saliva) without significant dietary alteration; unstimulated saliva flow > 0.2 ml/min
|
Moderate symptoms; oral intake alterations (e.g., copious water, other lubricants, diet limited to purees and/or soft, moist foods); unstimulated saliva 0.1–0.2 ml/min
|
Inability to adequately aliment orally; tube feeding or TPN indicated; unstimulated saliva < 0.1 ml/min
|
–
|
–
|
A disorder characterized by reduced salivary flow in the oral cavity
|
|
Dysphagia
|
Symptomatic, able to eat regular diet
|
Symptomatic and altered eating/swallowing
|
Severely altered eating/swallowing; tube feeding or TPN or hospitalization indicated
|
Life-threatening consequences; urgent intervention indicated
|
Death
|
Disorder characterized by difficulty in swallowing
|
|
Gingival pain
|
Mild pain
|
Moderate pain interfering with oral intake
|
Severe pain; inability to aliment orally
|
–
|
–
|
Disorder characterized by a sensation of marked discomfort in the gingival region
|
|
Lip pain
|
Mild pain
|
Moderate pain; limiting instrumental ADL
|
Severe pain; limiting self care ADL
|
–
|
–
|
A disorder characterized by a sensation of marked discomfort of the lip
|
|
Mucositis oral
|
Asymptomatic or mild symptoms; intervention not indicated
|
Moderate pain; not interfering with oral intake; modified diet indicated
|
Severe pain; interfering with oral intake
|
Life-threatening consequences; urgent intervention indicated
|
Death
|
A disorder characterized by inflammation of the oral mucosal
|
|
Oral cavity fistula
|
Asymptomatic; clinical or diagnostic observations only; intervention not indicated
|
Symptomatic; altered GI function
|
Severely altered GI function; TPN or hospitalization indicated; elective operative intervention indicated
|
Life-threatening consequences; urgent intervention indicated
|
Death
|
A disorder characterized by an abnormal communication between the oral cavity and another organ or anatomic site
|
|
Oral dysesthesia
|
Mild discomfort; not interfering with oral intake
|
Moderate pain; interfering with oral intake
|
Disabling pain; tube feeding or TPN indicated
|
–
|
–
|
A disorder characterized by a burning or tingling sensation on the lips, tongue or entire mouth
|
|
Oral hemorrhage
|
Mild; intervention not indicated
|
Moderate symptoms; medical intervention or minor cauterization indicated
|
Transfusion, radiologic, endoscopic, or elective operative intervention indicated
|
Life-threatening consequences; urgent intervention indicated
|
Death
|
Disorder characterized by bleeding from the mouth
|
|
Oral pain
|
Mild pain
|
Moderate pain; limiting instrumental ADL
|
Severe pain; limiting self care ADL
|
–
|
–
|
A disorder characterized by a sensation of marked discomfort in the mouth, tongue or lips.
|
|
Periodontal disease
|
Gingival recession or gingivitis; limited bleeding on probing; mild local bone loss
|
Moderate gingival recession or gingivitis; multiple sites of bleeding on probing; moderate bone loss
|
Spontaneous bleeding; severe bone loss with or without tooth loss; osteonecrosis of maxilla or mandible
|
–
|
–
|
A disorder in the gingival tissue around the teeth
|
|
Salivary duct inflammation
|
Slightly thickened saliva; slightly altered taste (e.g., metallic)
|
Thick, ropy, sticky saliva; markedly altered taste; alteration in diet indicated; secretion-induced symptoms; limiting instrumental ADL
|
Acute salivary gland necrosis; severe secretion-induced symptoms (e.g., thick saliva/oral secretions or gagging); tube feeding or TPN indicated; limiting self care ADL; disabling
|
Life-threatening consequences; urgent intervention indicated
|
Death
|
Disorder characterized by inflammation of the salivary duct
|
|
Salivary gland fistula
|
Asymptomatic; clinical or diagnostic observations only; intervention not indicated
|
Symptomatic; altered GI function; tube feeding indicated
|
Severely altered GI function; hospitalization indicated; elective operative intervention indicated
|
Life-threatening consequences; urgent operative intervention indicated
|
Death
|
Disorder characterized by an abnormal communication between a salivary gland and another organ or anatomic site
|
|
Tooth development disorder
|
Asymptomatic; hypoplasia of tooth or enamel
|
Impairment correctable with oral surgery
|
Maldevelopment with impairment not surgically correctable; disabling
|
–
|
–
|
A disorder characterized by a pathological process of the teeth occurring during tooth development
|
|
Tooth discoloration
|
Surface stains
|
–
|
–
|
–
|
–
|
A disorder characterized by a change in tooth hue or tint
|
|
Toothache
|
Mild pain
|
Moderate pain; limiting instrumental ADL
|
Severe pain; limiting self care ADL
|
–
|
–
|
A disorder characterized by a sensation of marked discomfort in the tooth
|
Table 5
Late oral complications post radiotherapy
|
Tissue involved
|
Type of cancer treatment
|
Oral complications
|
|||
|---|---|---|---|---|---|
|
CT
|
RT
|
HSCT
|
Surgery
|
||
|
Oral mucosa
|
X
|
X
|
X
|
Mucosal changes
|
|
|
X
|
X
|
Secondary oral infections
|
|||
|
X
|
Mucosal GVHD (lichenoid/plaque type)
|
||||
|
X
|
X
|
X
|
Secondary malignancy
|
||
|
Taste buds
|
X
|
X
|
X
|
Taste disorders
|
|
|
Salivary glands
|
X
|
X
|
X
|
Dry mouth
|
|
|
X
|
X
|
X
|
Sialoadenitis
|
||
|
X
|
Multiple mucocele
|
||||
|
X
|
X
|
X
|
Secondary oral infections
|
||
|
X
|
X
|
X
|
Secondary malignancy
|
||
|
Teeth
|
X
|
X
|
X
|
Rampant caries
|
|
|
X
|
X
|
X
|
Dental malformations
|
||
|
?
|
X
|
X
|
Cervical sensitivity
|
||
|
Periodontium
|
?
|
?
|
Advanced periodontitis
|
||
|
X
|
Desquamative gingivitis
|
||||
|
Connective tissue and musculature
|
X
|
X
|
X
|
Trismus
|
|
|
X
|
X
|
X
|
Loss of elasticity
|
||
|
X
|
X
|
X
|
Limited mouth opening
|
||
|
Bone (jaw)
|
X
|
Osteoradionecrosis
|
|||
|
X
|
X
|
Bisphosphonate-related osteonecrosis
|
|||
|
X
|
X
|
X
|
X
|
Developmental malformations
|
|
|
Nerve
|
X
|
X
|
X
|
X
|
Neuropathy and chronic pain
|
Table 6
NIH scale for oral cGVHD activity assessment
|
Mucosal change
|
No evidence of cGVHD
|
Mild
|
Moderate
|
Severe
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Erythema
Lichenhold
Ulcers
Mucocelesa
|
None
|
0
|
Mild enythema or moderate erythema (< 25 %)
|
1
|
Moderate (> 25 %) or Severe erythema (< 25 %)
|
2
|
Servere erythema (25 %)
|
3
|
||
|
None
|
0
|
Hyperkeratotic changes (< 25 %)
|
1
|
Hyperkeratotic changes (25–20 %)
|
2
|
Hyperkeratotic changes (50 %)
|
3
|
|||
|
None
|
0
|
None
|
0
|
Uicers involving (20 %)
|
3
|
Servere ulcerations (20 %)
|
6
|
|||
|
None
|
0
|
1–5 mucoceles
|
1
|
6–10 scattered mucocel3es
|
2
|
Over 10 mucoceles
|
3
|
|||
|
Total score for all mucosa chages
|
||||||||||
4.2 Oral Mucosal Alterations
The prominent effects of cancer treatment on the oral mucosa are seen in the early period following treatment and results in oral mucositis. Once mucositis has developed, the oral mucosa is more susceptible to the toxic effects of a second cycle of RT or CT (Sonis 2007). This clinical observation is supported by histomorphological evaluations of oral mucosal specimens taken from patients 6 to 12 months after RT. A decreased number of blood vessels, different expression patterns of adhesion molecules and subpopulations of integrins and macrophages are found (Prott et al. 2002). Patients complaining of a burning sensation months after their acute mucositis has apparently healed support this observation.
Targeted therapy was associated with various oral complications. Recently drugs belong to the Mammalian Target of rapamycin (mTOR) inhibitors were associated with aphthous-like oral lesions, mostly sirolimus, everolimus and temsirolimus. Other oral symptoms, such as dysphagia, dry mouth and dysguesia were reported in relation to mTOR inhibitors as well. Furthermore, additional groups of targeted therapies, such as monoclonal antibodies (cetuximab, bevacizumab) and tyrosine kinase inhibitors (sunitinib, sorafenib, and imatinib) were suggested to have oral adverse events.
Patients post allogeneic HSCT may develop oral mucosal GVHD. GVHD is an allo-immune inflammatory process, which results from a donor-origin cellular response against host tissues. The chronic form of GVHD (cGVHD) occurs in 30–50 % of allogneic transplants and oral involvement is seen in up to 80 % of them (Schubert and Correa 2008). The clinical presentation includes lichenoiod, erythematous, or ulcerative lesions which may be occasionally asymptomatic, but more often is associated with pain, burning sensations, or a rough texture of the oral surfaces. Painful mucosal lesions may represent a significant impediment to nutritional intake and performing oral hygiene.
Clinical examination of the oral tissues can support the diagnosis of GVHD. Correlation of oral changes with systemic manifestations should be considered, although the oral cavity may be the only site affected. Histopathologic evaluation may be complementary to clinical evaluation (Bradley et al. 2003). Scoring of oral cGVHD was addressed by the NIH in 2006 (Pavletic et al. 2006) (Table 6). Other scales may be used for research (Piboonniyom et al. 2005; Elad et al. 2003).
Importantly, GVHD is one of the potential risk factors associated with the development of secondary solid cancers including those presenting in the head and neck area (see “Secondary Malignancy”).
4.3 Taste Disorders
When the oral and pharyngeal mucosa is exposed to radiation, taste receptors are damaged and taste discrimination becomes increasingly compromised (National Cancer Institute 2008; Ruo Redda and Allis 2006; Gamper et al. 2012; Epstein et al. 2010). Loss of saliva flow (hyposalivation) impairs the transport and solubilization of gustatory stimulants to the taste buds, which leads to taste disorders. The outcome may be a reduction in taste sensitivity (hypogeusia), absence of taste sensation (ageusia), or a distortion of normal taste (dysgeusia). Studies in RT patients showed mixed results about the pattern of taste impairment. Some studies showed that sensitivity to sweet mainly decreases and that sensitivity to bitter increases. Others showed that sensitivity to bitter and salty simulants is mainly decreased (Ruo Redda and Allis 2006) and that umami was the only taste to be impaired (Shi et al. 2004).
After 3–4 weeks of radiation it is common for patients to complain of an absence of a sense of taste. It will generally take upwards of several months after the end of radiation therapy for taste receptors to recover and become functional, but it may require up to 2 years (Ruo Redda and Allis 2006).
Radioactive iodine in the form of (Paulino et al. 2000) I131 is used to treat thyroid cancer and reportedly causes taste alterations in approximately 10 % of patients (Rosenbluth et al. 2005, Lee JN et al. 2010).
Most commonly used cytotoxic agents have been linked to chemosensory disorders (Comeau et al. 2001; Bernhardson et al. 2007; Berteretche et al. 2004), and are known to damage chemosensory structures and disrupt saliva and mucus production, which indirectly affects the sense of taste (Schiffman and Zervakis 2002). Research into the side effects of cytotoxic chemotherapies indicates that 46–77 % of patients receiving chemotherapy report changes in taste (Lindley et al. 1999; Foltz et al. 1996; Rhodes et al. 1994; Wickham et al. 1999; Youngblood et al. 1994). However, most of these changes resolve within a few months after chemotherapy ends with little long-term impact (Bernhardson et al. 2007).
Approximately 20 % of patients 90–100 days post HSCT report moderate to severe levels of taste change (Epstein et al. 2002). More patients report reduced intensity of taste and abnormal tastes. However, it seems that these taste changes have a limited impact on QOL (Epstein et al. 2002). In patients 24–30 months post HSCT taste changes are also noted (Marinone et al. 1991). In patients that received allogeneic transplants, hypogeusia to salt and sour, but no difference in sweet or bitter are reported. In recipients of autologous transplants statistically significant taste changes are seen (Marinone et al. 1991). These finding are ambiguous as other studies show recovery in 80 % of patients 1 year-post HSCT (Mattsson et al. 1992). It is important to note that in pediatric patients undergoing HSCT taste changes may resolve faster (Cohen et al. 2012).
4.4 Salivary Gland Dysfunction
Long-term salivary gland dysfunction is a common complication of cancer treatment, predominantly in RT and in allogeneic HSCT patients. Salivary gland impairment is also well recognized following CT, and generally recovers within 6 months (Chaushu et al. 1995).
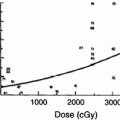
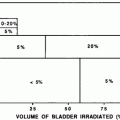
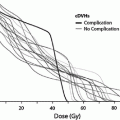
The RT-induced damage to the salivary gland results in a quantitative and qualitative change in saliva. Consequently, there is loss of salivary function, reduction in salivary flow and symptoms of dry mouth. The irradiated volume of salivary gland tissue correlates directly with the severity of oral complications (Cheng et al. 1981; Eisbruch et al. 1999; Roesink et al. 2001). The bottom range of the mean doses which have been found to cause significant salivary flow reduction is from 26 to 39 Gy (Roesink et al. 2001; Eisbruch et al. 2001). Changes in quantity of saliva occur shortly after radiotherapy. With cumulative RT dose of 60–70 Gy, more irreversible extensive damage to salivary flow rate is detected (Vissink et al. 2003). The implementation of alternative fractionation schedules, like hyper fractionation and accelerated fractionation has only a negligible positive effect on salivary gland function and morphology in animal models (Vissink et al. 2003; Price et al. 1995; Coppes et al. 2002). However, evidence in humans shows that xerostomia (a sensation of decreased saliva) is worse in accelerated fractionation compared to conventional fractionation (Awwad et al. 2002).
The introduction of IMRT allows more accurate delivery of a specific radiation dose to the tumor and spares the surrounding tissues, including major salivary glands. There is much encouraging data suggesting that salivary gland impairment is reduced by using IMRT compared to traditional radiotherapy techniques (Rathod et al. 2013). A systematic review concluded that the benefits of IMRT on salivary gland function, xerostomia, and xerostomia-related QOL are most pronounced late (≥ 6 months) after radiotherapy and there are improvements in xerostomia-related QOL over time (Jensen et al. 2010).
It is unclear yet what the sparing effect of novel radiation techniques such as cyberknife, protons and carbon ions is (Thariat et al. 2011).
Dry mouth in HSCT patients is a multifactorial condition. The most common etiologies are the conditioning regimen, chronic GVHD (cGVHD) and chronic administration of medication. In addition altered oral intake may be associated with dehydration.
(1)
The conditioning regimen: Salivary secretion is substantially reduced during the conditioning stage of HSCT (Chaushu et al. 1995). Gradual flow rate recovery typically begins a few days following HSCT. Patients conditioned with chemotherapy or chemotherapy and total lymph node irradiation display earlier and complete recovery of saliva secretions 2–5 months after engraftment. Recovery is delayed and incomplete when total body irradiation (TBI) is added to the conditioning regimen, suggesting that TBI induces irreversible damage to the parotid glands resulting in permanent hyposalivation (Chaushu et al. 1995).
(2)
GVHD: 60 % of cGVHD patients experience hyposalivation and a direct correlation is observed between the degree of hyposalivation and the severity of GVHD (Nagler et al. 1996; Alborghetti et al. 2005).
(3)
Chronic medication use: As the vast majority of HSCT patients remain under polypharmacy treatment, the added risk of drug-induced dry mouth, in addition to the impact of the cGVHD, exists.
Cancer treatment and cGVHD also result in a change of salivary composition. Clinically, saliva color becomes yellowish to brownish, mucoid, sticky and viscous. The qualitative changes in saliva include increased viscosity, reduced buffering capacity, altered salivary electrolyte concentrations, and increased levels of anti-bacterial proteins including sIgA, lysozyme and lactoferin (Vissink et al. 2003; Makkonen et al. 1986; Valdez et al. 1993; Izutsu et al. 1985; Hiroki et al. 1994; Nagler et al. 1996). When flow rates and buffer capacity is diminished, salivary pH is lowered. In the presence of food containing fermentable carbohydrates, plaque pH is decreased and the lack of clearance, due to decreased salivary flow, inhibits the rise of the plaque pH back to normal levels. The prolonged low pH environment impairs the balance between demineralization and remineralization predisposing to greater demineralization and results in dental caries. In the absence of preventive therapy, primarily fluoride, the dental caries progress very rapidly (see following paragraphs about “Dentition and Periodontium”). In addition, the acidic plaque pH provides optimal conditions for the shift of the oral flora to a cariogenic flora (Brown et al. 1975). Since the decrease in salivary flow rate is greater than the increase in immunoprotein and lysozyme levels, a significant immunoprotein deficit compromises saliva’s protective role (Kielbassa et al. 2006).
Patients complaining about xerostomia often describe that the discomfort increases during night and that adhesion of the tongue to the palate necessitates repeated arousal to drink water during their night sleep. This polydypsia lead to nocturnal polyuria. Clinical evaluation will reveal a dry appearance of the oral mucosa. In extreme oral dryness the oral mucosa may be atrophic, erythematous and fissured. Some patients may describe episodic painful swelling of the salivary glands, which is usually caused by retrograde infection due to the absence of the washing effect of saliva in the salivary ducts. When such an acute sialoadentitis exists, the salivary gland region may be swollen, the covering skin may be erythematous and tender, and salivary expression upon milking will be missing.
The typical manifestation of cGVHD involving the minor salivary glands is multiple mucoceles, with labial and soft-palate mucosa being the most frequently involved sites. Mucoceles often cause a disturbed sensation in the mouth but not pain (Schubert et al. 2004). In rare cases mucoceles may evolve into a large mucocele or ranule.
Hyposalivation has multiple additional consequences (Schubert and Correa 2008; Vissink et al. 2003
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree