© Springer International Publishing Switzerland 2017
Gabriele Tonni, Waldo Sepulveda and Amy E. Wong (eds.)Prenatal Diagnosis of Orofacial Malformations10.1007/978-3-319-32516-3_11. Orofacial Clefts in the Fetus: What We Know and What We Should Know
(1)
Department of Obstetrics and Gynecology, Prenatal Diagnostic Service, Guastalla Civil Hospital, AUSL Reggio Emilia, Reggio Emilia, Italy
(2)
Maternal-Fetal Diagnostic Center, Fetalmed, Santiago, Chile
(3)
Department of Maternal-Fetal Medicine, Palo Alto Medical Foundation, Mountain View, California, USA
1.1 Introduction
During embryogenesis, the development of the lips and palate begins at an early stage; the lips develop by week 4 postfertilization age, the primary palate fuses between 4 and 6 weeks, and the secondary palate is formed between 8 and 12 weeks. Cleft lip (CL) is the result of failure of the maxillary process to fuse with the medial nasal prominence, while a cleft in the secondary palate is the result of failure of the palatine process to elevate or grow [1]. Cleft palate (CP) originates at the uvula, causing uvula bifida, and progresses along the midline involving the soft palate or both the soft palate and the hard palate; in contrast, a cleft lip and cleft palate (CLP) starts at the lip and continues posteriorly involving the alveolar ridge, the hard palate, and the soft palate [2].
1.2 Epidemiology and Incidence
Cleft lip with or without cleft palate (CL/CLP) may occur as a result of a number of causes. Cigarette smoking during pregnancy has been documented to be associated with an increased chance of having a child with CL/CLP [3], although no consistent evidence between orofacial clefts with maternal exposure to ambient air pollutants can be documented [4].
Nonsyndromic cleft lip and cleft palate (NSCL/P) occurs with no positive family history in the vast majority of cases, although NSCL/P may be commonly found in cousins [5].
The prevalence of CL/CLP varies by ethnicity. In a large survey over 7.5 million births, the overall prevalence of CL/CLP was 9.92 per 10,000 (3.28 per 10,000 for CL and 6.64 per 10,000 for CL/CLP, respectively). Of these, 77% of CL/CLP were isolated, 16% had associated malformations, and 7.3% occurred as part of recognized syndromes [6]. The National Birth Defects Prevention Study (NBDPS), a large database used to identify genetic and environmental risk factors for birth defects, reported the prevalence of NSCL/CLP to be 0.3/1,000 live births for CL, 0.5/1,000 live births for CL/CLP, and 0.4/1,000 live births for CP. Cleft involvement was predominantly unilateral in cases of CL and CL/CLP, with left-sided cleft as the most frequent type observed. Orofacial clefts were isolated in 80% of cases and 25% had Pierre Robin sequence [7]. In Nova Scotia, the overall prevalence of orofacial clefts was 2.1 in 1,000 live births and, although no cases of isolated CP were detected prenatally, there was a trend towards an improvement in detection of CL/CLP over the years (from 14% during 1992–1996 to 30% during 1997–2002). Of cases of orofacial clefting in this study, 34% were associated with additional fetal structural malformations and 9.8% were associated with chromosomal abnormalities [8]. When considering the prevalence of orofacial clefts among the Asian population, the Chinese Birth Defects Monitoring Network reported prevalence rates of 14.2 per 10,000 live births for NSCL/P and 2.4 per 10,000 live births for syndromic CL/CLP, for an overall prevalence of orofacial clefting of 16.6 per 10,000 live births. In addition, this study showed that CL/CLP varied by gender, urban–rural classification, and geographic location when compared to cleft palate, particularly for nonsyndromic cases [9]. These findings were also confirmed by the study of Johnson et al. [10].
1.3 Genetics of Orofacial Clefts
NSCL/P has a multifactorial etiology that includes both genetic and environmental factors. In a European genome-wide association study, susceptibility loci for NSCL/P have been identified on chromosomes 8q24, 10q25, and 17q22. In addition, the IRF6 (interferon regulatory factor 6) gene has been demonstrated to be a genetic risk factor for NSCL/P, particularly in northern Europe [11–14]. Moreover, MSX1 (muscle segment homeobox) and TGFB3 (transforming growth factor beta-3) genes may be involved in the pathogenesis of clefting. In a non-Caucasian population, TGFA (transforming growth factor alpha) has shown to play less of a role than it does in Caucasians in cases of NSCL/P [15,16]. Different orofacial cleft (OFC) loci have been mapped on chromosome 6p24 (OFC1), 2p13 (OFC2), 19q13.2 (OFC3), and 4q (OFC4). OFC5-8 are identified by mutations in the MSX1, IRF6, PVRL1 (poliovirus receptor-like 1 or nectin-1, responsible for cleft lip/palate-ectodermal syndrome and Tessier cleft palate type 7), and TP73L (tumor protein) genes, respectively. OFC9 maps to 13q33.1-q34, whereas OFC10 is secondary to mutation haploinsufficiency of the SUMO1 (small ubiquitin-like modifier 1) gene located on 2q33.1.
In addition, MTHFR, TGF-beta3, and RAR alpha play a role in cleft development, and the TBX22 gene located on Xp21.1 is responsible for cleft palate with ankyloglossia and is involved in cases of isolated CP [17].
1.4 Associated Fetal Malformations
Orofacial clefts can be associated with other structural fetal malformations, chromosomal abnormalities (orofacial clefts are seen in 40.7% of trisomy 13 cases and 6.9% of trisomy 18 cases) [18], or genetic syndromes. The incidence of associated structural abnormalities is reported to vary with the type of cleft: 9.8% in cases of unilateral CL/CLP, 25% in cases of bilateral CL/CLP, and 100% of cases of midline CL/CLP [19–22]. When CL/CLP occurs in isolation, there does not appear to be an increased risk of chromosomal abnormalities in fetuses [23]; for example, Gilham et al. [24] reported no karyotype abnormalities in over 200 cases of isolated unilateral or bilateral CL/CLP. However, in a series by Chmait et al.[25], 22% of cases of CL/CLP that were presumed to be isolated prenatally were found to have an additional anomaly after delivery, which must be taken into account when counseling parents regarding the utility of invasive amniocentesis and neonatal prognosis.
Orofacial clefts are also associated with first-trimester findings, occurring in 19.5 per 1,000 live births with an enlarged nuchal translucency (NT). The relative risk of an isolated or non-isolated cleft in a fetus with enlarged NT is 8 and 53, respectively [26].
1.5 Accuracy of 2D and 3D Ultrasound in the Prenatal Detection of CL/P: First Versus Second Trimester of Pregnancy
The accuracy of detecting orofacial clefts has changed dramatically over the past 20 years with the trend toward improved detection in recent years. It should be kept in mind that CL is associated with CP in approximately 80% of cases [27]. In a French study, the detection rates increased from approximately 5% in the early 1980s to over 26% in the late 1990s [28], while in Norway, the detection rate was as high as 58% in the late 1990s to 2004 [21]. In Western Australia, the detection rate for CL/CLP was reported to be 22.2% from 1996–2003, with no detection prior to 15 weeks of gestation [29]. The detection rate was almost similar in cases of unilateral CL/P (40.6%) and bilateral (44.4%); although the detection rate for isolated CL was 33.3%, no cases of isolated CP were diagnosed by prenatal ultrasound. Bister et al. [30] reported antenatal ultrasound to have a higher detection rate of specifically CL/CLP of 93%, although the sensitivity of ultrasound for the detection of all types of orofacial clefts, including isolated CL and isolated CLP, was only 65%. However, sensitivity was 100% with no cases of false positives.
1.6 The Role of 2D Ultrasound
The prenatal detection rate of isolated CP when only 2D ultrasound is used is typically very poor, ranging from 0 to 1.4% [21,22,24,31,32]. For example, the Eurofetus group reported the sensitivity of 2D ultrasound to diagnose orofacial clefts at routine scan to be 25%, 22% for CL/CLP, and 1.4% for isolated CP [32] depending on the experience and training of the examiner. Brohnstein et al. [33] diagnosed CL/CLP in only 0.07% of cases using transvaginal ultrasound between 12 and 16 weeks of gestation with a false-negative rate of 8%, while Jones reported a higher detection rate (14–25%) of CL/CLP [34]. According to Maarse et al. [35], the diagnostic accuracy of second-trimester transabdominal 2D ultrasound at detecting orofacial clefts in low- and high-risk populations ranged from 9–100% for CL/CLP, 0–22% for CP only, and 0–73% for all types of orofacial clefts. In contrast, 3D ultrasound in high-risk women resulted in a detection rate of 100% for CL, 86–90% for CL/CLP, and 0–89% for CP only.
1.7 The Role of 3D Ultrasound
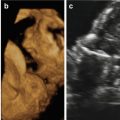
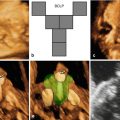
Although facial clefting of the fetus can be prenatally diagnosed by 2D ultrasound, the introduction of 3D ultrasound together with the development of new software applications has led to new insights into the prenatal ultrasound study of the fetal palate. There is now a growing body of evidence that 3D ultrasound may enhance the prenatal visualization of the fetal face and hence the detection of orofacial clefting, especially if 3D ultrasound is performed as a targeted examination in cases of suspected clefting after 2D ultrasound. The best time frame for ultrasound-based screening is 18–23 weeks of gestation [1].
For this purpose, several techniques have been developed such as the “flipped-face” view [36], the “reverse-face” view [37], the Faure technique and “angle insonation” [38], the “oblique-face” view [39], and the “retronasal triangle (RNT)” view [40]. The RNT view was specifically developed to analyze the primary palate during the first-trimester scan.
Martinez-Ten et al. [36] have reported that the “oblique-face” view appears to be the best method when the secondary palate is involved; this view correctly identified involvement of the hard palate in 100% of cases, compared to the 71% detection rate of the “reverse-face” view and 86% detection rate of the “flipped-face” view. Shadowing from the surrounding bony structures and the fetal tongue may limit the study of the degree of extension of the cleft to the posterior palate [41]. The diagnosis of CL/CLP may be improved by the use of 3D ultrasound in surface mode [42–45]. Campbell and Lees [46] have demonstrated that 3D ultrasound using the “reverse-face” view may enhance sensitivity by examining the fetal face initially in the frontal plane and subsequently rotating 180° on the vertical axis to examine the secondary palate. 3D ultrasound may be clinically useful in the visualization and reconstruction of the fetal primary and secondary palate, especially in cases in which 2D ultrasound is limited by acoustic shadowing [1,36,47]. When 2D ultrasound is complemented by 3D applications, compared with 2D ultrasound alone, the prenatal diagnosis of CP is improved from 22 to 89% [47].
1.8 The Role and Value of Ultrasound-Targeted MRI (Magnetic Resonance Imaging)
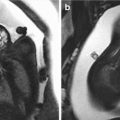
Ultrasound-targeted fetal magnetic resonance imaging (MRI) may be a useful integrated diagnostic tool for ultrasonographically suspected CL/CLP [41,48–50]. MRI can evaluate the anterior six tooth buds and the horseshoe-shaped curve bony structure of the tooth-bearing alveolar ridge better than ultrasound [51]. While the sagittal plane is useful in the study of the hard and soft palates, the coronal plane remains the best plane for diagnosis of abnormalities of the nose and lips [52].
Isolated clefts of both the soft and hard palate are more easily detected by real-time MRI [53]; a positive predictive value of 96% and negative predictive value of 80% have been reported [54]. Using T2-weighted half-Fourier acquisition single-shot turbo spin echo (HASTE) sequence using sagittal, coronal, and axial planes, Wang et al. [55] reported that 91% of cleft palates were correctly detected. In a study by Mailáth-Pokorny et al. [56], fetal MRI successfully visualized a cleft in the primary and secondary palates in 100% of cases, especially in the axial and coronal planes. MRI is less dependent on examiner expertise, maternal habitus with increased body mass index, severe oligohydramnios, and unfavorable fetal position than ultrasound.
Conclusions
- 1.
Orofacial clefts are one of the most common congenital anomalies, with a variable prevalence ranging between 1:500 and 1:1,000 live births.
- 2.
The number of infants with clefts in a population can vary according to maternal age, maternal race/ethnicity, genetic predisposition, socioeconomic factors, and environmental factors during intrauterine life, such as smoking and alcohol.
- 3.
At least 15% of fetuses with CL/CLP have other associated anomalies, such as structural malformations, chromosomal abnormalities, and/or genetic syndromes.
- 4.
CL/CLP can be diagnosed at the time of first-trimester screening, as diagnostic criteria have now been established especially for 3D ultrasound.
- 5.
2D ultrasound evaluation of the face and upper lip should become an integrated part of the of second-trimester scan.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree