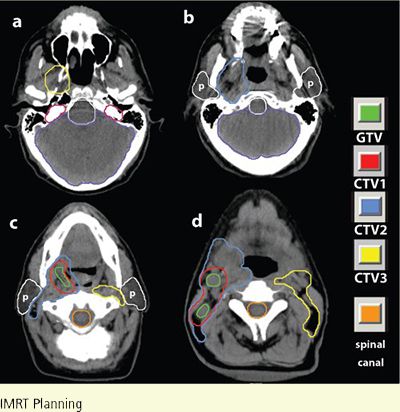
FIGURE 8-6. CTV1, CTV2, and CTV3 delineation in a patient with clinically T2N2bM0 squamous cell carcinoma of the tonsil who received definitive IMRT. For clarity, the parotid glands are marked with lower case p.
1. ANATOMY
• The oropharynx is the posterior continuation of the oral cavity; it communicates with the nasopharynx superiorly and the laryngopharynx inferiorly. It can be subdivided into the palatine (faucial) arch and oropharynx proper. Its subsites include the base of tongue (BOT), palatine tonsils, tonsillar pillars, soft palate, and the pharyngeal wall.
1.1. Tonsillar Fossa and Faucial Arc
• The palatine arch, a junctional area between the oral cavity and the laryngopharynx, is formed by the soft palate and the uvula superiorly, the anterior tonsillar pillar and glossopalatine sulcus laterally, and the glossopharyngeal sulcus and the BOT inferiorly.
• Figure 8-1 shows a coronal section of the oropharynx with relationships in the parapharyngeal regions.
• The retromolar trigone has been included in the structures of the faucial arch, although it is actually located within the oral cavity. Its apex is in line with the tuberosity of the maxilla (behind the last upper molar); the lateral border extends superiorly into the buccal mucosa; medially, it blends with the anterior tonsillar pillar; and its base is formed by the distal surface of the last lower molar and the adjacent gingivolingual sulcus.
• The lateral walls of the oropharynx are limited posteriorly by the tonsillar fossa and posterior tonsillar pillar (pharyngopalatine folds). These pillars are folds of mucous membrane that cover the underlying glossopalatine and pharyngopalatine muscles. Deep into the lateral wall of the tonsillar fossa are the superior constrictor muscles of the pharynx, upper fibers of the middle constrictor, pharyngeus and stylopharyngeus muscles, and glossopalatine and pharyngopalatine muscles. The tonsillar fossa continues into the lateral and posterior pharyngeal walls.
• The tonsillar fossa and faucial arch have a rich submucosal lymphatic network, laterally grouped into four to six lymphatic ducts that drain into the subdigastric, upper cervical, and parapharyngeal lymph nodes. Submaxillary lymph nodes may be affected in lesions involving the retromolar trigone, the buccal mucosa, or even the BOT.
1.2. Base of Tongue
• The BOT is bounded anteriorly by the circumvallate papillae, laterally by the glossopharyngeal sulci and oropharyngeal walls, and inferiorly by the glossoepiglottic fossae or valleculae and the pharyngoepiglottic fold.
• The vallecula is the transition zone between the BOT and the epiglottis and is considered a part of the BOT.
• The surface of the tongue is irregular because of the submucosal lymphoid follicles, but the mucous membrane is smooth when compared with the dorsum of the oral tongue. The lymphoid tissue at the BOT does not penetrate the intrinsic tongue muscles.
• The BOT is almost parallel to the posterior pharyngeal wall. Its musculature is continuous with that of the oral tongue and the floor of the mouth anteriorly. The genioglossus fibers fan out in the tongue to interdigitate with the intrinsic tongue musculature (Fig. 8-2). The tongue base also is continuous with the pre-epiglottic space.
2. NATURAL HISTORY
2.1. Tonsil
• Many tonsillar tumors are keratinizing squamous cell carcinomas, which can be graded I to IV, depending on the degree of differentiation.
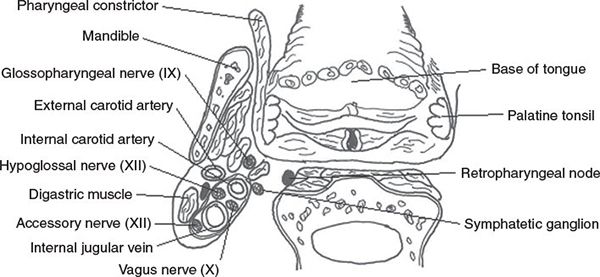
FIGURE 8-1. Coronal section of the oropharynx showing relationships in the parapharyngeal regions.


FIGURE 8-2. (A) Sagittal section of the upper aerodigestive tract. (From Respiratory System. Anesthesia Technician & Technologist’s Manual, Lippincott Williams & Wilkins, 2008.) (B) Sagittal magnetic resonance imaging (MRI). Lymphoid tissue (LT) at the tongue base does not penetrate the intrinsic tongue muscles (IM); it is limited to the surface. Genioglossus fibers fan out in the tongue (arrowheads) to finally interdigitate with the intrinsic tongue musculature. The tongue base is continuous with the pre-epiglottic space (arrow). Areas of high signal intensity within soft palate (SP) are the result of its fatty content. (From Million RR, Cassisi NJ, Mancuso AA. Oropharynx. In: Million RR, Cassisi NJ, eds. Management of Head and Neck Cancer: A Multidisciplinary Approach, 2nd ed. Philadelphia, PA: JB Lippincott, 1994:402, with permission.)
• Carcinomas arising in the faucial arch tend to be keratinizing and more differentiated than those of the tonsillar fossa.
• Tonsillar fossa lesions tend to be infiltrative, often involving the adjacent retromolar trigone, soft palate, and BOT. Perez1 reported that the primary tumor was confined to the tonsillar fossa in only 5.4% of 384 patients; 65% had involvement of the soft palate, and 41% had extension into the BOT.
• Tumors of the faucial arch can be superficially spreading, exophytic, ulcerative, or infiltrative; the last two types are frequently combined. They become extensive and involve the adjacent hard palate or buccal mucosa in <20% of patients.1
• Tumors of the tonsillar fossa have a high incidence of lymph node metastases (60% to 70%); most are in the subdigastric lymph nodes, midjugular chain, and submaxillary lymph nodes (in lesions extending anteriorly); 5% to 10% involve the posterior cervical lymph nodes (Table 8-1).1
• Metastases in the low cervical chain occur in approximately 5% to 15% of patients with upper cervical lymph node involvement.1
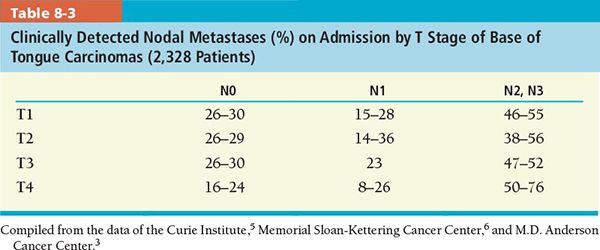
• The incidence of metastatic lymph nodes in the neck increases with tumor stage. Less than 10% of T1 lesions, 30% of T2 lesions, and 65% to 70% of T3 and T4 lesions have metastatic cervical lymph nodes at presentation.1
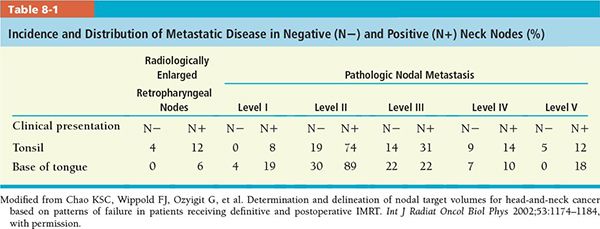
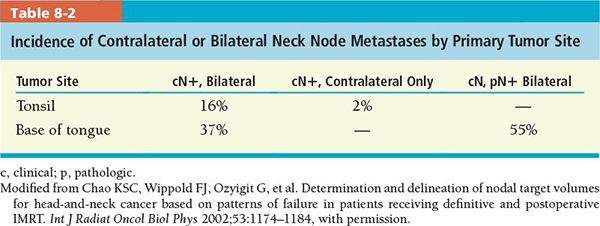
• Contralateral lymphadenopathy in tonsillar tumors is noted in 10% to 15% of patients with positive ipsilateral lymph nodes, more frequently if the primary tumor extends to or beyond the midline (Table 8-2).1
• Tonsillar pillar and soft palate lesions have an overall metastatic rate of approximately 45%. Initially, the most frequent site of nodal involvement is the jugulodigastric lymph nodes. Approximately 10% of patients have submaxillary lymph node involvement. Tumors of the retromolar trigone, anterior faucial pillar, and soft palate rarely metastasize to the posterior cervical lymph nodes. Contralateral spread is infrequent (10%).1
2.2. Base of Tongue
• Squamous cell carcinoma of the BOT tends to have early, silent, and deep infiltration; therefore, it is difficult to estimate tumor extension by clinical examination. However, tumors originating from the peripheral regions usually remain there.2–4
• BOT cancers have little tendency to spread to the palatine tonsils, whereas tonsillar cancers tend to invade the BOT.
• Vallecular lesions are often exophytic and invade along the mucosa to the lingual surface of the epiglottis, laterally along the pharyngoepiglottic fold, and then to the lateral pharyngeal wall and anterior wall of the pyriform sinus.
• The first echelon nodes are the subdigastric (level II) nodes; lymphatic drainage then continues along the jugular chain to the mid- and lower jugular nodes. If anterior extension into the oral tongue or massive upper neck disease is present, then the submandibular lymph nodes may be involved. The posterior cervical lymph nodes are often involved, but submental spread is rare.
• Bilateral and contralateral lymphatic spread is common (Fig. 8-3A,B). Retrograde spread to retropharyngeal lymph nodes has been reported particularly in advanced lesions. Typical spread of disease by T stage is illustrated in Figure 8-3C.
• The deeply infiltrating nature of BOT cancers correlates with the high frequency of lymphatic metastases at presentation (80% of patients overall, with bilateral spread in 37% to 55%).2–6
• The incidence of clinically positive neck node at presentation is approximately 50% to 83% (Table 8-3).
• The incidence of pathologically positive neck node in clinically N0 neck is approximately 22% to 33%. Contralateral lymphatic metastasis at presentation is 37% (Table 8-2).2–4 Table 8-1 shows the percentage of the incidence and distribution of metastatic disease in clinically negative and positive neck nodes in BOT cancers.
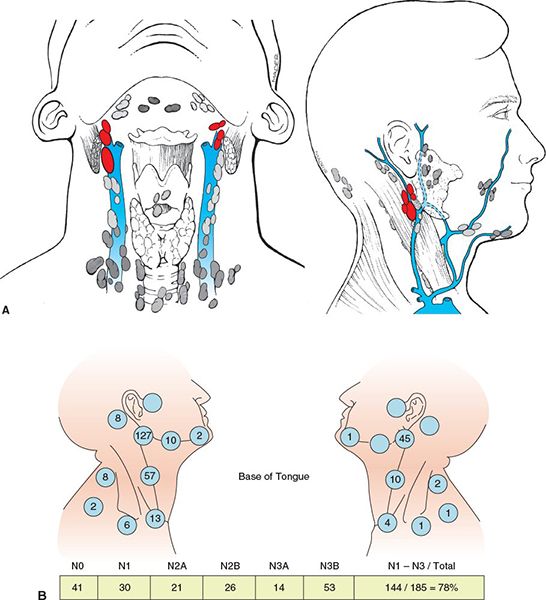
FIGURE 8-3. (A) Lymphatics of head and neck. The red node highlights the sentinel node, which is the jugulodigastric node. Left: anterior view; right: lateral view. (From Rubin P, Hansen JT. TNM Staging Atlas with Oncoanatomy, 2nd ed. Philadelphia, PA: Lipppincott Williams & Wilkins, 2012:78. Modified from Agur AMR, Dalley AF, eds. Grant’s Atlas of Anatomy, 12th edition. Philadelphia: Lippincott, Williams & Wilkins, 2009.) (B) Distribution of nodal involvement at presentation of squamous cell carcinoma of base of the tongue (BOT). (Redrawn from figure in Lindberg RD. Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tract. Cancer 1972;29:1446–1449, with permission.)
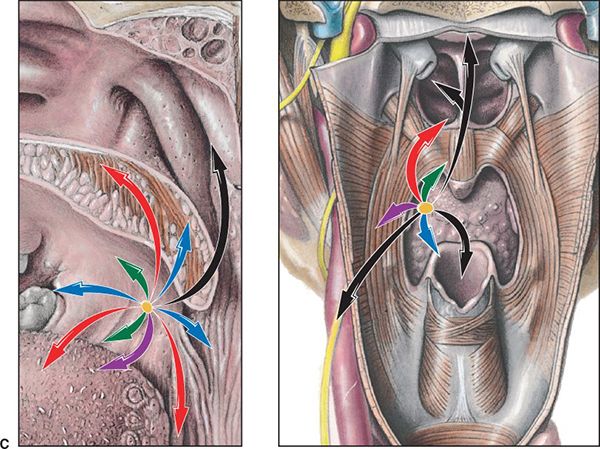
FIGURE 8-3. (C) Typical patterns of spread in oropharyngeal cancer. Left: Sagittal view: highlights cancer spread from oropharynx into nasopharynx and hypopharynx. Right: Coronal view: indicates the sphincter musculature of the pharynx: middle constrictor between superior constrictor of the nasopharynx and inferior constrictor of the hypopharynx as to invasive pathways of pharyngeal tube. The primary cancer (oropharynx) invades in various directions, which are shown as color-coded vectors (arrows) representing stages of progression: Tis, yellow; T1, green; T2, blue; T3, purple; T4a, red; and T4b, black. (From Rubin P, Hansen JT. TNM Staging Atlas with Oncoanatomy, 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2012:73. Modified from Agur AMR, Dalley AF, eds. Grant’s Atlas of Anatomy, 12th edition. Philadelphia: Lippincott, Williams & Wilkins, 2009.)
3. DIAGNOSIS AND STAGING SYSTEM
3.1. Signs and Symptoms
3.1.1. Tonsil
• Sore throat is the most frequent symptom. Asymptomatic lesions are frequently found on routine examination.
• Dysphagia or otalgia is related to the anastomotic-tympanic nerve of Jacobson.
• Trismus may be a late manifestation if the masseter or pterygoid muscle is involved.
• Invasion of the tongue will eventually limit tongue mobility. Ulceration at the junction of the anterior tonsillar pillar and oral tongue can cause a great deal of pain.
• Tumors of the tonsillar fossa have similar signs and symptoms, except that the lesions tend to be larger before symptoms develop. Ipsilateral sore throat and otalgia are the hallmarks of these lesions.
• Lymphomas of the tonsil tend to be large submucosal masses but may ulcerate and appear similar to carcinomas.
3.1.2. Base of Tongue
• The BOT is visualized only by indirect mirror examination, so earlier diagnosis is rare. The earliest symptom is mild sore throat. Many of the early lesions are asymptomatic and relatively silent, and a subdigastric neck mass is frequently the first sign.
• Necrosis and internal bleeding may cause sudden enlargement and mild tenderness.
• Dysphagia, a nasal quality to the voice, and deep-seated otalgia occur with enlargement of the mass. Otalgia is associated with tumor involvement of the retropharyngeal space.
• Hypoglossal nerve invasion is rare but can cause unilateral paralysis and atrophy of the tongue when it does occur.
3.2. Physical Examination
3.2.1. Tonsil
• Indirect mirror examination and digital palpation are required for diagnosis.
• In addition to a complete history and physical examination, a complete examination of the head and neck is mandatory. Fiberoptic nasopharyngolaryngoscopy is routinely used to examine the upper aerodigestive tract. Speech and swallow evaluation may be indicated prior to treatment.
3.2.2. Base of Tongue
• Early lesions are usually submucosal and relatively soft. Because the surface of the BOT is irregular, it is difficult to palpate the mass. Rigid or flexible endoscopes permit examination in some patients.
• Palpation through the lateral floor of the mouth can be helpful to detect anterior extension.
• Fullness in the soft tissue around the hyoid bone may be a sign of inferior penetration through the valleculae.
• Fixation of the tongue causes incomplete protrusion of the fused site.
3.3. Imaging
• The main imaging tools of the oropharyngeal region are computed tomography (CT) and magnetic resonance imaging (MRI).
• CT is better for the lymph nodes and bone detail, so it is the preferred initial study. MRI is used adjunctively.
• Positron emission tomography (PET) may be used as a complementary imaging modality for determination of target volumes that appear equivocal on CT or MRI. However, potential drawbacks of PET, including poor spatial resolution (~6 to 8 mm), partial volume effects causing blurring of the edges, and reported sensitivity of only 50% to 70% in the clinically negative neck,7 have put into question its reliability as a primary imaging tool for target delineation.
• Lee and co-workers105 used F-18-Misonidazole for PET imaging of hypoxia and found that the presence of PET-avid tissues did not correlate with clinical outcome for patients treated with platinum-based chemotherapy and IMRT.
• A recent study by Shukla-Dave et al.9 in 74 squamous cell carcinoma patients using dynamic contrast-enhanced MRI indicated that the parameter Ktrans (volume transfer constant) was a strong predictor of outcome for those with stage IV disease.
• Evaluation of the BOT should include slices from the nasopharynx to the lower neck. Axial sections are often sufficient; however, coronal sections may be required when lesions invade the base of the skull. Sagittal MRI is necessary for detection of early pre-epiglottic space invasion.
• CT and MRI sections of this region require injection of contrast media. Contiguous 3- to 4-mm slices should be used through the primary tumor and neck. It is important to keep the field of view small so that the pictures are magnified and spatial resolution is optimized. Evaluation of the BOT must include a complete CT study of the cervical and retropharyngeal nodes (Fig. 8-4).
• CT and MRI are excellent for showing the deep structures surrounding the pharynx. The deep tissue planes are generally symmetric, and obliteration of the deep fat spaces, such as the parapharyngeal space, or invasion of deep musculature is a sign of spread. Such spread is frequently not expressed as signs and symptoms nor detected by physical examination. The pharyngeal wall becomes tightly surrounded by musculature, and the intervening fat planes are less visible lower in the oropharynx, making diagnosis of invasion more difficult.
• Lymphoid tissue is present throughout the oropharynx and is responsible for most of the variation seen in the surface contours on CT and MRI. Inexperienced interpreters may frequently mistake the various bumps and bulges on the mucosal surfaces for tumors. These regions should either be ignored or be used as prompts to look for adjacent deep infiltration as a sign of pathology. These surfaces are best evaluated by physical examination, not by CT or MRI. There are no findings that can distinguish lymphoid tissue and other benign mucosal lesions from cancer other than infiltration of the deeper structures.
• Any tumor that is suspicious for deep infiltration should be studied primarily with CT. A significant portion of MRI studies in this area will be of low quality because of motion artifacts. In general, MRI is preferred for the evaluation of the parapharyngeal space.
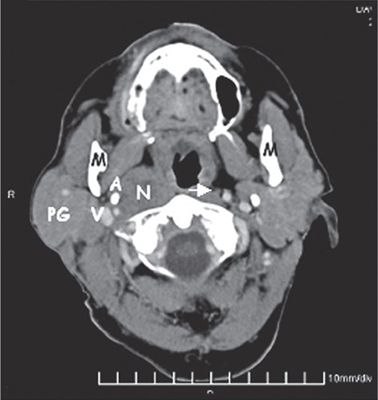
FIGURE 8-4. Computed tomography (CT) study shows an enlarged retropharyngeal lymph node (N). The node lies medial to the carotid artery (A), parotid gland (PG), and jugular vein (JV). A more normal-sized retropharyngeal node (arrow) is present on the opposite side. M, mandible.
• The relationship of tumor margins to both the lingual neurovascular bundles can be anticipated to be visualized on imaging with far greater precision than on physical examination. Occasionally, retrograde spread of tumor out of the tongue along the lingular neurovascular bundles to the external carotid artery will be visible.
• Tumors in the region may also grow onto the styloid musculature. Inferior growth along the mylohyoid and hyoglossus muscles may bring the tumor to the insertion of these muscles on the hyoid bone, and there may be direct extension into the soft tissues of the suprahyoid and infrahyoid neck at that point. Occult spread from the BOT to the pre-epiglottic space may also be visualized.
3.4. Staging
• In 2010, the American Joint Committee on Cancer (AJCC) issued a new (seventh) edition of its Cancer Staging Manual10; the readers are referred to this new edition for more details on staging. The most significant change from the sixth edition was that T4 lesions have been split into two subgroups, one for moderately advanced local disease (T4a) and the second for very advanced local disease (T4b).
4. PROGNOSTIC FACTORS
4.1. Tonsil
• Stage: The stage of primary tumor and presence of involved cervical lymph nodes have a significant correlation with local control and survival.11–16
• Treatment-Related Factors: External-beam dose and fractionation schedule also significantly influence local control.14 Cause-specific survival rate is influenced by planned neck dissection.14
• Histologic Differentiation: Multivariate analysis revealed that survival was significantly influenced by histologic differentiation.14
• HPV Status: Infection with human papilloma virus (HPV) has been identified as a prognostic factor associated with favorable outcomes in patients with cancers of the oropharynx. The highest prevalence of HPV is found in tonsillar carcinomas.16 In a retrospective analysis of RTOG 0129, patients with HPV-positive tumors, treated with chemoradiotherapy, had a 58% reduction in risk of death, as compared with the HPV-negative cohort.17 A review of SEER data by Nguyen et al.18 showed that younger patients had a better prognosis, and this could be due to higher HPV infection rates.19,20
4.2. Base of Tongue
• Tumor Size and Extent: BOT cancers have a worse prognosis than those observed in oral tongue cancers because of larger sizes at diagnosis, more frequent spread to adjacent structures, and higher rate of lymphatic spread. However, stage for stage, they may have a prognosis similar to that of oral tongue cancers.21
• Stage: One of the most dominant prognostic factors is tumor stage.21
• Overall Treatment Time: Survival and locoregional control become worse when overall treatment time increases.21
• Histopathologic Grade: Poorly differentiated or undifferentiated carcinomas are shown to have better survival and local control rates.
• HPV Status and Tobacco Use: The presence of HPV infection has been identified as a favorable prognostic factor; however, the risk of death in HPV-positive patients increases with each additional pack-year of tobacco smoking.17
• Molecular Markers: Increased expression levels of HPV p16 protein correlate with better disease-free survival and overall survival outcomes, while increased expression of epidermal growth factor receptor (EGFR) is associated with poorer prognosis.22,23
• Other: Other prognostic factors include age and extension to both epilarynx and endolarynx (associated with poor survival).5,24
4.3. Oropharynx General
• Kong et al.19 reported on correlations between HPV status and other molecular markers: it was prognostic for survival and correlated inversely with EGFR expression but directly with T-cell infiltration. Moeller et al.25 used FDG-PET/CT and plain CT to assess post-RT mortality risk in 98 patients, 79% of whom had oropharyngeal cancer. Both imaging modalities had high specificity and negative predictive value to separate patients into high- and low-risk groups. Notably HPV-negative status and disease outside the oropharynx correlated with poorer outcome. Moeller et al.20 later reported that Ku80 overexpression was an independent predictor of locoregional failure and mortality. HPV-negative patients with Ku80 overexpression had 9 times higher mortality at 2 years.
• Calvin et al.26 examined tissue from 450 patients previously enrolled in RTOG 90–03 for RT in locally advanced head and neck cancers, 60% of which were in the oropharynx. Somewhat counterintuitively they found that high microvessel density did not correlate with outcome.
• Chen et al.27 examined 101 matched pairs of head and neck cancer patients, about half of whom had their lesions located in the tonsil or BOT. One of the pair continued using tobacco during radiation therapy and the other had ceased tobacco use. As might be expected, both 5-year overall survival and locoregional control rates were significantly lower in the patients who continued to smoke (23% vs 55% and 58% vs 69%, respectively).
• Overexpression of EGFR was a significant predictor for lowered overall survival and higher locoregional relapse in data from 533 head and neck cancer patients (60% oropharynx). Chung et al.28 concluded that pretreatment EGFR expression data could be useful in patient stratification.
5. GENERAL MANAGEMENT
• Surgery and radiation are equally successful in controlling early stage oropharyngeal cancer. Radiation may be the preferred modality where the functional deficit will be high, such as the tongue base or tonsil. A total of 175 patients with AJCC stages I and II oropharyngeal carcinoma were treated with external-beam radiotherapy at M.D. Anderson Cancer Center.29 The actuarial 5-year locoregional control and disease-free survival rates were 81% and 77%, respectively.
• The management of advanced-stage carcinomas of the oropharynx requires a multidisciplinary approach to establish optimal treatment. In general, the preferred treatment has been to combine surgery with postoperative radiation therapy. Recent studies have explored concurrent chemoradiotherapy as an alternative approach for treatment of advanced-stage disease with a view to organ preservation.30 Aggressive radiation therapy alone will give control rates equivalent to those of surgery for cancers originating in the tonsil.
• A recent study by Soltys et al.31 including 60 patients with locally advanced N2 or N3 oropharyngeal disease using induction and concurrent chemoradiation showed that partial neck dissection (PND) offered no significant benefit to those achieving clinically complete response. Prognosis for partial responders was worse, and PND was indicated.
5.1. Tonsillar Fossa and Faucial Arc
5.1.1. Tumors of Tonsillar Fossa
• Tables 8-4 and 8-5 show the initial local control rates for carcinoma of the tonsil according to T stages with different treatment strategies.
• Table 8-6 summarizes the disease-specific survival of patients with carcinoma of the tonsil.
• T1 or T2 lesions can be treated with irradiation or surgery alone.
• T1, T2, and T3 tumors are treated with irradiation alone (66 to 75 Gy in 6 to 7 weeks, depending on stage; conventional prescription to primary tumor and gross adenopathy is 70 Gy in 2 Gy/fraction). Regional lymph nodes are treated with 44 to 75 Gy, depending on nodal involvement. Uninvolved nodal stations are considered as either low- or intermediate-risk, depending on proximity to gross disease areas, and are treated to 44 to 64 Gy in 1.6 to 2 Gy/fraction. Interstitial brachytherapy has been used to deliver an additional dose (25 to 30 Gy) to the primary tumor.32
• In T3 and T4 tumors, a combination of irradiation and surgery has been advocated because of the higher incidence of recurrences with either modality alone.12,13,33
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree