Citation
Observation
Types of patients
Bonfiglio [10]
Thrombosed arteries
Dysbaric osteonecrosis
Ficat and Arlet [1]
Arteriolar thromboses
Etiology not noted
Spencer and Brookes [11]
Thrombus observed in multiple intraosseous vessels of one patient; associated with degenerative changes of the blood vessels within the femoral head
5 corticosteroid-treated (for 3 months to 4 years after transplant) transplant recipients
Saito et al. [12]
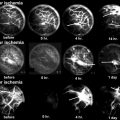
One figure of a Haversian vessel (arrow), showing damaged vascular wall and thrombosis in the lumen; fibrosis in the Haversian canal
Osteonecrosis (1° and 2°)
Asherson et al. [13]
Vasculitis and arterial thrombosis
Lupus patients
Jones [14]
Focal anemic infarctions associated with subchondral necrosis and adjacent extravasation of blood into fatty marrow
1. Kissing bug
2. Trauma
3. Alcohol-associated ON
Cheras et al. [15]
“Established” intraosseous thrombosis of the femoral head
Various etiologies
Egan and Munn [16]
Widespread infarction of the marrow elements and bony trabeculae; multiple thrombin thrombi in nasal septum
Antiphospholipid antibody syndrome (case report)
Starklint et al. [17]
(a) Vessels packed with erythrocytes and fibrin thrombi
(c) Osteonecrosis (1°and 2°); 14 femoral heads from 12 patients
Laroche et al. [18]
(b) Vascular thrombosis in femoral heads of 4 of 20 patients
(d) Osteonecrosis (1° and 2°)
Thrombosed arterioles but fewer than in their animal models. Based on their observations, they have concluded that arteriopathy is “essential”
2° Osteonecrosis
Aaron [21]
Microcirculatory thrombus in the subchondral cancellous bone
2° ON
Many of the comorbidities associated with osteonecrosis are also associated with vascular pathologies (Table 10.2). For example, systemic lupus erythematosus (SLE) is associated with vasculitis, premature atherosclerosis, and hypercoagulability. The incidence of thrombosis in patients with SLE is 2 per 100 person-years of follow-up [39]. There are also several reports of intraosseous thrombi in ON cases associated with dysbarism [40], antiphospholipid antibody syndrome [41, 42], and Crohn’s disease [43, 44]. It should be noted here that many of these conditions result in corticosteroid therapy. The association of corticosteroids to ON will be discussed thoroughly later in the chapter.
Table 10.2
Abnormal factor levels (serology)
Patients | s-tPA-Fx | PAI-Fx | Lp(a) | RAPC | aCL | Protein C | Protein S | HCY | aPL | Factor VIII | Factor V Leiden | vWF | Apo B | Homocysteine | AT III (Ag and fct) | Beta-globulin | plasminogen | Anti-p53 | crosslaps | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Asherson et al. [13] | 37 ON | X | ||||||||||||||||||
38 knee; 63 hip | x | |||||||||||||||||||
Cenni et al. [26] | 36 ON | X (smokers + I ON) | x | X | ||||||||||||||||
Chotanaphuti et al. [20] | 55 ONFH | x | x | x | ||||||||||||||||
Glueck et al. [27] | 30 ON;18 2° ON; 12 I ON | x | x | X (I ON) | ||||||||||||||||
Glueck et al. [28] | 31 ON; 13 2° ON; 18 I ON | x | x | x | x | |||||||||||||||
Glueck et al. [29] | 50 ONFH | x | x | |||||||||||||||||
Glueck et al. [31] | 36 ONFH | x | x | x | x | |||||||||||||||
Glueck et al. [32] | 133 ON; 62 2° ON; 71 I ON | X (I ON) | X (2° ON) | X | X (2° ON) | |||||||||||||||
Gruppo [33] | 55 ON of the jaw | X | X | X | X | X | ||||||||||||||
Jones et al. [34] | 45 ONFH | x | x | x | ||||||||||||||||
Korompilias et al. [35] | 40 ONFH | X | ||||||||||||||||||
Korompilias et al. [36] | 216 ONFH | x | x | x | ||||||||||||||||
Pósán et al. [9] | 49 adults | x | X | |||||||||||||||||
Tan et al. [37] | 12 ONFH | X | X | X | X | |||||||||||||||
Zalavras et al. [38] | 68 ON; 17 I ON; 51 2° ON | X | X | X (2° ON) | X | X (I ON) | X (also α1 & α2) |
10.3 Coagulation Cascade and Regulation
Every basic physiology and hematology textbook describes the physiologic mechanisms involved in the formation and the resolution of blood clots [45, 46]. While it is not the intention of this chapter to provide a detailed description of the pathways involved (e.g., extrinsic vs. intrinsic pathways), it is important to understand the complexity of the coagulation factors involved in both coagulation and fibrinolysis.
Blood clots form via one of the two pathways based upon whether the inciting event is tissue factor (TF) pathway (extrinsic) or amplification by the formation of a primary complex of factors in contact with collagen in the endothelium of damaged vessel walls (intrinsic) [45]. While some of the factors involved in the cascades are the same (e.g., factor X), others are different [factor VII (extrinsic) vs. factor XII (intrinsic)] (Fig. 10.1). In both pathways, the final step is the conversion of prothrombin to thrombin by prothrombin activator in the presence of calcium ions. Thrombin converts fibrinogen to fibrin monomers, which, in turn, polymerize into long fibrin threads. The clot acts to entrap platelets, blood cells, and plasma [48]. A thrombus is established when a blood clot attaches and remains stationary along the wall of a blood vessel, impairing blood flow and, in some cases, totally obscuring lumen [48]. The downregulation of coagulation is effected by proteolytic inactivation of specific coagulation factors [49]. These factors include antithrombin III, protein C, protein S, and others.
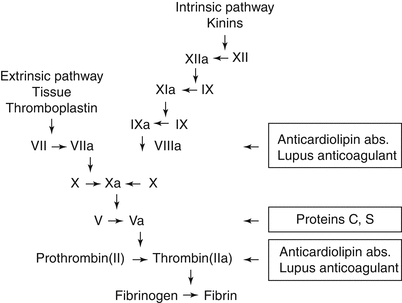

Fig. 10.1
The intrinsic and extrinsic coagulation pathways (Published with permission Aaron and Ciombor [47])
As with the coagulation cascades, the fibrinolytic system is built upon a system of activators and inhibitors [50]. The induction of fibrinolysis is dependent on the formation of the active enzyme, proplasmin, a serine protease that degrades fibrin clots. Plasminogen is released as a zymogen and converted to plasmin by one of two activators: tissue plasminogen activator (tPA) and urokinase plasminogen activator (u-PA) – based upon whether fibrin is in the circulation or cell bound, respectively. Inhibitors of the fibrinolytic system include α2-macroglobluin (A2M) and α1 antitrypsin as well as the plasminogen activator inhibitors (PAI-1 and PAI-2) [51].
10.4 Osteonecrosis and Coagulation Factors
The literature is replete with reports of an association between osteonecrosis and abnormalities of the factors participating in both the coagulation and fibrinolytic pathways. Many studies have focused on the circulating levels of these factors, while others have evaluated whether there is an association with inherited thrombophilia or hypofibrinolysis. While a genetic abnormality may lead to unusually high or low circulating levels of a specific coagulation factor, this may not always be the case. It is, therefore, important to conduct both genetic and serologic studies to explore this association. Several external factors such as corticosteroid therapy and alcoholism have also been associated with abnormal circulating levels of these factors and increased risk of thromboembolism [52–54]. Unfortunately, many of these associations have been based on case reports or small series [14, 55–67]. The following discussion will address the results of the larger studies.
10.4.1 Thrombophilia
Thrombophilia is the increased tendency towards intravascular thrombosis [28]. Elevations in anticardiolipin antibodies, anti-β-GPI antibodies, homocysteine and activated protein C resistance have been observed with thrombophilia [34, 46, 68]. Inherited thrombophilia is associated with mutations in the genes for factor V (factor V Leiden, a mutation in the F5 gene at position 1691), prothrombin (prothrombin G20210A, at position 20210 in the 3′ untranslated region of the gene), factor XIII, methylenetetrahydrofolate reductase (MTHFR), and abnormal fibrinogen [46, 68]. When factor V is mutated, it is unable to bind to protein C so that factor V is more resistant to inactivation by protein C [28, 47]. Deficiencies in antithrombin III, protein C, or protein S are also associated with thrombophilia [28, 46]. As proteins C and S are involved in the activation of factor V, decreased levels of these proteins result in an increased tendency to form thrombi [47]. As shown in Tables 10.2 and 10.3, numerous reports have indicated an association between osteonecrosis and abnormal levels of specific factors or gene mutations involved with thrombophilia.
Table 10.3
Genes associated with hypofibrinolysis and thrombophilia
Protein encoded | Gene(s) | Coagulopathy | References |
|---|---|---|---|
Plasminogen activator inhibitor-1 | 4G/4G; 4G/5G alleles in the promoter region | Hypofibrinolysis | Glueck et al. [29] |
Glueck et al. [31] | |||
Ferrari et al. [69] | |||
Plasminogen activator inhibitor-1 | SNP rs1799889 (promoter region; −675, 4G/5G), rs2227631, (promoter region; −844 G/A), and rs11178 (3′ UTR region, +10700C/T) | Hypofibrinolysis | Kim et al. [70] |
Methylenetetrahydrofolate reductase | C677T polymorphism | Thrombophilia | Glueck et al. [31] |
Factor V Leiden | Mutation of F5 | Thrombophilia | Glueck et al. [71] |
Factor V Leiden | Arg506Gln mutation | Thrombophilia | Gillespie et al. [72] |
Prothrombin | 20210A gene mutation | Thrombophilia | Bjorkman et al. [24] |
Glueck and colleagues have studied the association between osteonecrosis and coagulopathies for over 20 years. They have reported higher resistance to activated protein C [28], low protein C [28], low protein S [30, 31], high anticardiolipin antibodies [34], high homocysteine levels [30, 31, 34], and high factor VIII [30]. Korompilias et al. have also reported increased resistance to activated protein C, high levels of anticardiolipin antibody, and low levels of protein S in some osteonecrosis patients [35, 36]. Deficiencies in antithrombin III, protein C, and protein S are associated with deep venous thrombosis of the extremities [73].
Heritable thrombophilia has also been investigated, especially in regard to mutations in genes for MTHFR, V Leiden, and prothrombin. The mutant form of MTHFR, the C677T polymorphisms, decreases the activity of the enzyme and interferes with the intracellular metabolism of homocysteine, resulting in an elevated plasma homocysteine level [74, 75]. Elevated levels of homocysteine are associated with thrombophilia [76]. As shown by Glueck et al. [31], osteonecrosis patients were more likely to have the heterozygous for the MTHFR mutation (64 %) than were controls (38 %) (p = 0.014). This was associated with elevated homocysteine levels in this patient cohort (p = 0.05). Resistance to activated protein C (RAPC) is associated with the Arg506Gln mutation in factor V (factor V Leiden) [24]. In a study of 68 patients with osteonecrosis, Björkman and colleagues reported 13.2 % of the patients with the factor V Leiden mutation, while the idiopathic group exhibited a 22.9 % rate [24]. In a study of 244 ON patients, Glueck et al. found that 9.4 % of the patients were heterozygous for factor V Leiden; the rates were similar for both idiopathic (9.3 %) and secondary (9.6 %) osteonecroses [71].
Thrombin plays a critical role in the conversion of fibrinogen to fibrin in the coagulation pathway (Fig. 10.1). Prothrombin is enzymatically cleaved by activated factor X to form thrombin, and this action is further enhanced in the presence of factor V. The mutation in prothrombin (20210A) causes increased circulating levels and therefore predisposes to thrombotic events [24, 75]. While Glueck et al. found no difference in the presence of prothrombin gene heterozygosity between osteonecrosis cases (3.4 %) and controls (2.9 %) [71], Björkman et al. reported a 5.9 % incidence of heterozygosity for the prothrombin 20210A mutation [24].
10.4.2 Hypofibrinolysis
Markers of hypofibrinolysi s have also been identified in some patients with osteonecrosis (Tables 10.3 and 10.4). Plasminogen activator inhibitor-1 (PAI-1) acts to inhibit tissue plasminogen activator (tPA) which is an activator of plasminogen (Fig. 10.2). When plasminogen becomes activated, it is converted to plasmin which then acts to dissolve the fibrinogen fibers within a blood clot (fibrinolysis). An increase in PAI-1 activity suppresses the generation of plasmin resulting in hypofibrinolysis [47]. Several studies have documented elevated levels of plasminogen activator inhibitor 1 (PAI-1) and/or low levels of tissue plasminogen activator [27–29, 31, 33, 34, 86].
Table 10.4
Risk factors for osteonecrosis associated with thrombophilic and hypofibrinolytic abnormalities
Lupus | |
|---|---|
Sickle cell | Nsiri et al. [79] |
Caisson disease | Candito et al. [80] |
Alcoholism with liver disease | Tran-Thang et al. [81] |
Inflammatory bowel disease | Koutroubakis et al. [82] |
End-stage renal disease/renal transplant
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|