CHAPTER 42 Other Infections of the Brain
ENCEPHALITIS
HIV Encephalitis
Epidemiology
Approximately 40 million people worldwide are infected with HIV. HIV-associated dementia (HAD) is now the most common cause of dementia worldwide among people aged 40 years or younger. Potent antiretroviral therapies have now reduced the incidence of HAD to as low as 10.5%.1 Combinations of antiretroviral substances have been shown to increase the CD4+ lymphocyte count and to decrease viral replication in plasma and lymph nodes to undetectable levels.
Clinical Presentation
HIV infection may lead to motor and cognitive deficits. The motor symptoms are usually mild and may manifest as a slowing of repetitive movements or difficulty with balance. Disabling HIV dementia presents as a slow decline in a patient’s cognitive abilities with a characteristic triad of cognitive, behavioral, and motor dysfunctions.2 Nearly 50% of HIV patients in the United States demonstrate neuropsychological testing performance that is below expectations compared to matched normative groups. HIV-associated neurocognitive disorders (HAND) can be subclassified into asymptomatic neurocognitive impairment (ANI), mild cognitive disorder (MND), and HIV-associated dementia (HAD).
Pathophysiology
HIV enters the central nervous system (CNS) early in the course of infection, possibly by using a cloak of human proteins to sneak into the cells (Trojan horse “stealth entry”).3 The brain then serves as an important reservoir for autonomous, selfsustaining, and persistent infection. Within the brain the virus resides primarily within microglia and macrophages.4
Pathology
Two neuropathologic consequences of cerebral HIV infection are multinucleated giant cell encephalitis (MGCE) and progressive diffuse leukoencephalopathy (PDL).5 MGCE and PDL may be two ends of a broad spectrum of morphologic changes induced by HIV. MGCE is characterized by perivascular accumulation of inflammatory cells, predominantly microglia cells, monohistiocytes, and macrophages. In PDL, diffuse loss of myelin and axons with reactive astrocytosis and distinctive multinucleated giant cells are seen in the deep white matter.5 New variants of HIVE have been reported recently, including severe leukoencephalopathy with intense perivascular macrophage and lymphocyte infiltration and chronic “burnt out” forms of HIVE.
Imaging
CT
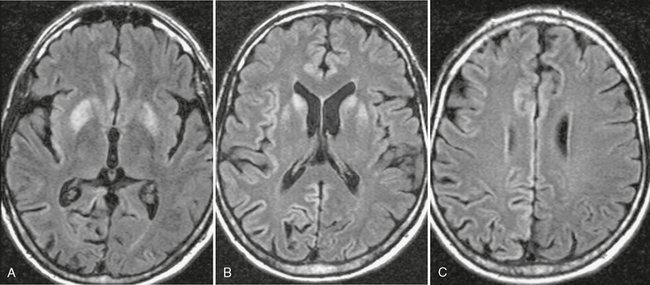
The most common CT finding in HAD is cerebral atrophy, which may be central, peripheral, or mixed.6,7 An early CT study of 200 patients reported that 37.5% of AIDS patients present with cerebral atrophy.8 Other CT studies of HAD show progression of the atrophy over time. Cortical atrophy is found in 85% of symptomatic patients, suggesting that cortical atrophy may be relatively specific to patients with neuropsychological impairment. CT may also show hypodensities in the white matter bilaterally (Fig. 42-1).
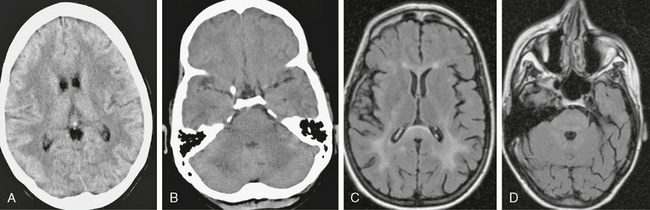
FIGURE 42-1 HIV encephalopathy. This young HIV-positive patient had a high viral load level in the plasma and the CSF and a low CD4+ T lymphocyte count. A and B, Noncontrast CT scans show bilateral hypodensity in the white matter and in the posterior fossa. No enhancement was present on postcontrast scans (not shown). C and D, Axial FLAIR-TSE MRI (TR/TE/TI 7385/130/2100) scans show bilateral, symmetric high signal intensity abnormalities in the periventricular white matter (C) and brain stem (D). Enlarged ventricles and sulci are noted. Neuropsychological examinations were consistent with subcortical dementia.
MRI
MRI has documented reduction of gray matter volume in the basal ganglia and posterior cortex and generalized loss of volume in the white matter.9
White matter lesions are found in about 80% of HAD patients (range: 43%-100%) and usually assume any of four patterns: diffuse, patchy, focal, and punctiate.10 These lesions are usually isointense or minimally hypointense on T1-weighted (T1W) imaging, have high signal on T2-weighted (T2W) imaging, and show no mass effect or enhancement (see Fig. 42-1). Two distinct patterns are observed on T2W imaging: (1) diffuse bilateral symmetrical high signal intensity in the white matter appears to represent HIV encephalopathy (see Fig. 42-1) and (2) patchy bilateral lesions with high T2 signal intensity in the gray and white matter appear to represent HIV encephalitis.10 High signal intensity lesions may also be seen in the splenium of the corpus callosum and in the crura of the fornices.11 Long-term studies show progressive increase in the white matter lesions with disease progression.12–14 Fluid-attenuated inversion recovery (FLAIR) sequences are superior to T2W images for detection of white matter lesions, especially in periventricular and subcortical locations.15 1H MR spectroscopy (MRS) is being used increasingly to detect early CNS involvement by HIV. Decreased N-acetyl-aspartate (NAA) and elevated choline (Cho) and myoinositol (MI) levels (lower NAA/creatine [Cr] ratio, increased Cho/Cr ratio, increased MI/Cr ratio) in the basal ganglia or frontal white matter are seen in early HIV infection of the brain.16 In HAD patients, perfusion MRI shows a statistically significant decrease in regional cerebral blood flow (rCBF) in the inferior lateral frontal cortices bilaterally with an increase in rCBF in the posteroinferior parietal white matter bilaterally.17
MRI and MRS have now been used in the clinical management of patients with HAD who are receiving potent antiretroviral therapy.18–20 A combination of antiretroviral drugs in patients with HAD may result in stabilization or even regression of white matter signal intensity abnormalities observed on MR images.19,20 The progression of white matter lesions on initial follow-up studies is likely the result of postinflammatory reactions due to immune reconstitutive effects after the initiation of highly active antiretroviral therapy (HAART).20,21 Despite potent therapies, the neuronal damage appears to progress without clinical manifestations and the progression of cerebral atrophy is apparent on MR images.20
Progressive Multifocal Leukoencephalopathy
Epidemiology
The JC virus was isolated in 1971 and named for the first patient in whom this virus was isolated.22 Approximately 5% (0.7%-11%) of HIV patients will develop PML during the course of their illness.23,24 PML may also occur in immunosuppressed patients with malignant diseases. Two cases of PML have been described recently in patients with multiple sclerosis who were treated with the α4-integrin inhibitor natalizumab.25
Clinical Presentation
Common manifestations of PML include weakness, gait abnormalities, speech disturbance, cognitive disorders, headache, and visual impairment. Without treatment, the prognosis for PML is usually poor, with death occurring after 2.5 to 4 months. Only a small number of cases (7%-9%) have been reported to have a more benign clinical course with prolonged survival (>12 months) and associated improvement in clinical and radiographic abnormalities in the absence of specific therapy. About half the AIDS patients with PML will not experience benefit from HAART. However, PML can also develop in AIDS patients who are undergoing HAART.26
Pathology
Demyelinated areas are recognized as discolorations of the white matter.
The histopathologic hallmark of PML is demyelination with enlarged oligodendroglial nuclei and bizarre astrocytes.27 The central portion of the lesion is characterized by an almost total breakdown of the myelin sheath and by axonal damage, with an increase in the extracellular space; the most medial area of lesion development consists of oligodendrocytes with nuclear inclusions and partially destroyed myelin, with a relative sparing of axons.
PML is usually multifocal, and the lesions may occur in any location in the white matter, thalamus, brain stem, and cerebellum. In rare cases, PML may be limited to the posterior fossa.28
Imaging
CT
On CT, PML lesions are recognized as multifocal hypodense lesions without mass effect or enhancement (Fig. 42-2).
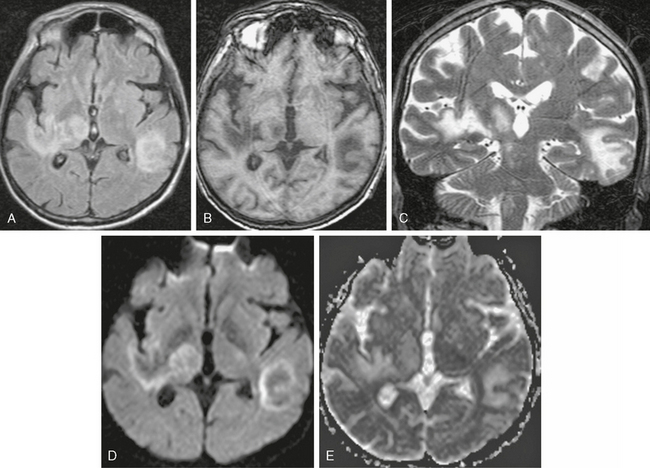
FIGURE 42-2 Progressive multifocal leukoencephalopathy (PML). This AIDS patient presented to the emergency department with impaired consciousness. A, Axial FLAIR MRI (TR/TE/TI 6000/100/2000) shows multiple, scalloped, high signal intensity lesions in both cerebral hemispheres and in the right thalamus. B, On noncontrast T1W TFE MRI (TR/TE/flip° 20/2.1/35°), the lesions have markedly low signal. C, Coronal T2W MRI shows the scalloped pattern of the lesions caused by involvement of the arcuate subcortical “U fibers.” D and E, Trace DWI images (D) and ADC map (E) show that the lesions have low signal centers with high signal margins (D), indicating elevated diffusivity in demyelinated PML lesions and cytotoxic edema along the active margins.
MRI
On T2W MR images, PML lesions are patchy, scalloped, high signal intensity white matter lesions with extension along the white fibers (Fig. 42-3).24,28 Subcortical arcuate fibers are involved, mass effect is mild or absent, and peripheral, faint enhancement is a rare feature.29–31 On T1W images, the PML lesions are markedly hypointense.24,28 Magnetization transfer ratios (MTRs) can be used to differentiate between PML and lesions in HAD. The mean MTR value of PML lesions is significantly lower than that of HAD lesions (26.1% vs. 47.9%).32 On DWI, different signal behavior between the lesion center and the extending margin has been observed (Fig. 42-4).33 On apparent diffusion coefficient (ADC) maps, signal intensity was elevated in the central area, whereas at the lesion margins two areas were distinguished: (1) a newer portion, with reduced water diffusibility compatible with cytotoxic edema and (2) less recent portions with intermediate values.33
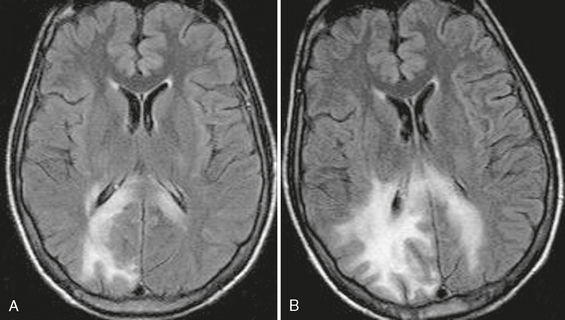
FIGURE 42-3 Progressive multifocal leukoencephalopathy (PML). This HIV-positive patient did not respond well to highly active antiretroviral therapy (HAART). A, Axial FLAIR MRI (TR/TE/TI 6000/100/2000) shows scalloped high signal intensity lesions in the right occipital lobe with extension into the corpus callosum. PML was diagnosed with positive polymerase chain reaction for JC virus in the CSF, and HAART was initiated. B, Follow-up MR examination 6 weeks after HAART showed worsening of the abnormal signal intensity. The patient died 4 months after the initial diagnosis.
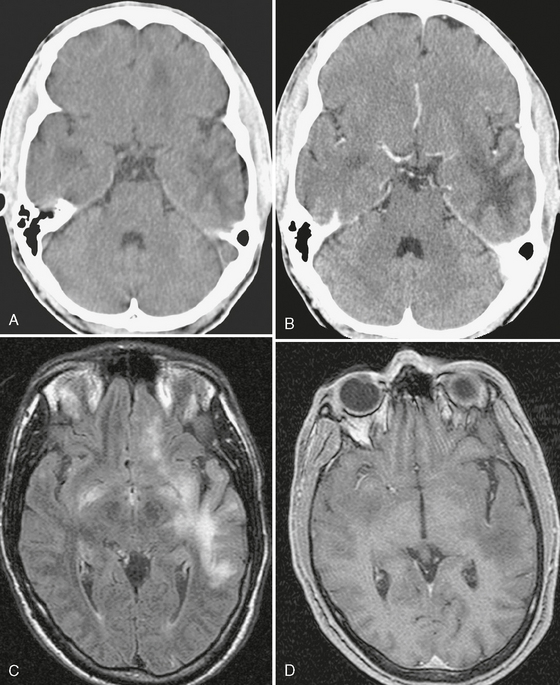
FIGURE 42-4 Progressive multifocal leukoencephalopathy (PML) in an AIDS patient. The initial noncontrast (A) and contrast-enhanced CT scans (B) show multiple, scalloped, hypodense, nonenhancing lesions in the temporal lobes and left frontal lobe. C, FLAIR-TSE MRI (TR/TE/TI 7373/130/2100) shows abnormal high signal intensity in the left hemisphere, with no mass effect. (D) The T1W TFE MRI (TR/TE/flip° 10/3.5/10°) shows a low signal lesion with no enhancement. The CSF gave a positive polymerase chain reaction for JC virus.
The 1H spectra of PML lesions are characterized by significantly reduced NAA, lactate presence, and increased choline and lipids.34,35 A decrease in NAA is the result of axonal loss, and the presence of lactate is related to cellular hypoxia. The increase in choline and lipid may reflect an accumulation of myelin breakdown products.
Although at present there is no specific therapy for PML, recent studies have shown clinical and radiologic improvement in patients with PML who underwent HAART.36–38 In patients with prolonged survival regression or stabilization, MRI findings paralleled the suppression of virus replication and immune response recovery. Worsening of the MRI findings, with development of contrast enhancement, mass effect, and edema may be seen in some patients.39 This phenomenon is known as immune reconstitution syndrome (IRIS) and is the result of a post-treatment inflammatory reaction due to the immune reconstitutive effect. Atrophic changes and increased hypointensity on T1W images with concomitant low signal on FLAIR images in these patients represent leukomalacia and burnt-out PML lesions.
Cytomegalovirus Infection
This infection is caused by cytomegalovirus (CMV), a member of the herpesvirus family.
Epidemiology
According to published studies, 40% to 100% of the general population are infected with CMV. CMV infections have been described with increasing frequency in patients with AIDS, with up to 30% of patients showing neuropathologic evidence of CMV infection.40 The incidence has decreased by more than 80% since the introduction of HAART.41
Clinical Presentation
Five distinct neurologic syndromes due to CMV infection have been described: retinitis, myelitis/polyradiculopathy, diffuse micronodular encephalitis, ventriculoencephalitis, and mononeuritis multiplex. In CMV infection, meningoencephalitis presents as fever, confusion, headache, and progressive dementia. Patients with ventriculoencephalitis have a more acute onset, with death usually occurring quickly. The infection of the CNS is difficult to diagnose while the patient is alive because CMV is difficult to culture from the cerebrospinal fluid (CSF). The recent development of the polymerase chain reaction technique has allowed isolation of CMV based on the presence of DNA within the CSF.42
Pathology
Enlarged cells with distended nuclei that contain viral inclusions called “owl’s eyes” are a pathologic hallmark of CMV encephalitis. Diffuse micronodular CMV encephalitis resembles HIV encephalitis histologically. Small microglial nodules and inclusion-bearing cytomegalic cells are widely distributed in the cortex, basal ganglia, brain stem, and cerebellum. In an autopsy series of 30 cases of CMV encephalitis, 76% of the patients had microglial nodules containing inclusion-bearing cells and 24% had CMV inclusions outside the nodules.43 In ventriculoencephalitis, periventriculitis, and ependymal and subependymal necrosis, inclusion-bearing CMV cells are observed.44
Imaging
CT
Generalized atrophy is the most commonly reported CT abnormality, but it is a nonspecific finding often seen in AIDS patients.45
MRI
The most common imaging findings in patients with CMV encephalitis are cortical atrophy, periventricular enhancement, and diffuse white matter abnormalities.45 Periventricular enhancement is not pathognomonic; it has been described in cases of lymphoma, toxoplasmosis, and other infections. White matter disease occurs as a result of inflammation of subependymal region and spread of the infection to the adjacent astrocytes of the white matter with infectious demyelination.46,47 In six patients with pathologically confirmed CMV infection of the CNS, Hassine and associates found atrophy in three patients, subependymal nodular lesions without enhancement in two patients, and ventriculitis in one patient.48
Rarely, cerebral mass lesions due to CMV could be observed in AIDS patients. In one study, two cases of cerebral mass lesions due to CMV were described.49 In both cases, a large contrast-enhancing mass was seen in the frontal lobe, with surrounding edema. CMV infection can also be present as choroid plexitis. In one reported case, a contrast-enhanced CT scan showed marked enhancement of the slightly enlarged right plexus. MRI confirmed the findings and the absence of enhancement of the ependyma.50 The discrimination between HIV and CMVassociated CNS disease is often difficult using clinical and imaging findings. MRS could be potentially useful in such cases. In one study, MRS was used to distinguish HIV encephalitis from CMV encephalitis.51 The findings suggest that a larger choline signal and a smaller NAA signal could be inferred within the white matter abnormalities due to HIV encephalitis/encephalopathy compared with CMV encephalitis.
Prion Diseases
Epidemiology
Prion diseases are rare, with fewer than 2 per million individuals affected per year.52 The elderly population is most commonly affected, with a peak incidence between 60 and 64 years of age. Prion diseases have also been demonstrated in young individuals.53 The sporadic form of CJD seems to affect females more often than men (ratio: 2 : 1).54,55 The majority of cases of CJD (about 85%) occur as sporadic disease, and a smaller proportion of patients (5%-15%) develop CJD because of inherited mutations of the prion protein gene. These inherited forms include Gerstmann-Sträussler-Scheinker syndrome and fatal familial insomnia.
Clinical Presentation
The sporadic form of CJD is characterized by rapidly progressive dementia. The patients suffer from myoclonic movements and both pyramidal (Babinski sign) and extrapyramidal symptoms (rigor, akinesia, choreatic movements).56 Isolated cerebellar symptoms as early manifestations occur in only 5%.55 The clinical features of vCJD include younger age at onset of disease and longer disease duration. Periodic sharp waves will be detected on electroencephalograms in cases of sCJD and will often be absent in vCJD cases. Common symptoms and signs in fatal familial insomnia include intractable insomnia, dysfunction of the autonomic system (hyperthermia, hypertension, tachycardia, tachypnea, hyperhidrosis), dementia, and motor paralysis.
Brain biopsy or autopsy is required to confirm the diagnosis. The detection of 14-3-3 protein in the CSF, an elevated concentration of neuron-specific enolase (NSE), and periodic sharp wave complexes on an electroencephalogram, along with clinical signs, usually allow for a probable diagnosis while the patient is still alive.57–59
Pathology
sCJD
The neuropathologic hallmark of sCJD is the highly diseasespecific spongiform change in brain tissue accompanied by neuronal loss and astrogliosis and microgliosis. Spongiform changes are characterized by diffuse or focally clustered small round or oval vacuoles in the neuropil of the deep cortical layers, cerebellar cortex, or subcortical gray matter, which might become confluent.60
vCJD
The florid plaque is a neuropathologic hallmark of vCJD.61 Widespread accumulation of PrP(res) will be detected on immunocytochemistry. Spongiform changes are most marked in the basal ganglia, whereas the thalamus exhibits severe neuronal loss and gliosis in the posterior nuclei.61
Fatal Familial Insomnia
In fatal familial insomnia, amyloid plaques are found in the thalamus and spongiotic changes are evident in the cortical areas. There is atrophy of the thalamus (with the mediodorsal and anterior ventral areas being more affected), and, with involvement of inferior olive, isolated cortical foci of spongiosis are present.
Imaging
MRI
sCJD
Typical MRI findings in sCJD are hyperintensity on T2W and FLAIR images in the cortex and basal ganglia (Fig. 42-5). Increased signal on diffusion-weighted imaging (DWI) and a low apparent diffusion coefficient (ADC) in the basal ganglia and cerebral cortex are also common.62 These findings are more frequently seen in the head of the caudate nuclei, the putamen, the thalamus, the striatum, and the cortical gray matter. It has been speculated that the increased signal on DWI is related to decreased water diffusion by the spongiform changes. A decrease in NAA and elevation of MI have been demonstrated in the frontal white matter in a patient with fCJD and in the parietal and frontal white matter in a case of sCJD using a short echo time and a single-voxel technique.63,64 Metabolic abnormalities have also been demonstrated in the pulvinar of the thalamus, with decreased NAA and significantly increased MI in vCJD.65,66
fCJD
MRI findings are quite variable but seem similar to those in sCJD, with signal abnormalities on T2W, FLAIR, and DWI in gray matter structures, such as the basal ganglia, the insula, and the cerebral cortex. Hyperintensity lesions on T1W imaging in the globus pallidus have also been reported both in fCJD and also in a case of sCJD.67
vCJD
The characteristic MRI abnormality in vCJD is hyperintensity on T2W sequences in the pulvinar nuclei, called the “pulvinar sign.”68 MRS findings in vCJD are characterized by marked metabolic changes in the thalamus, decreased NAA, and increased MI, reflecting the pattern of gray matter signal abnormalities seen on T2W and FLAIR images.65,69
Gerstmann-Sträussler-Scheinker Disease
In a patient with GSS, a reduced NAA/Cr ratio was found in the frontal cortex, the putamen, the cerebellar vermis, and in both hemispheres.69
Rasmussen’s Encephalitis
Clinical Presentation
Patients frequently have episodes of epilepsia partialis continua and, much less frequently, generalized status epilepticus. The seizures are intractable despite aggressive medical management. Patients demonstrate a progressive motor deficit and mental deterioration that occur over several years. Most individuals with Rasmussen’s encephalitis will experience frequent seizures and brain damage over the course of the first 8 to 12 months and then enter a phase of permanent, but stable, neurologic deficits. On electroencephalography, persistent temporal delta activity is observed early on, with multifocal spikes and background slowing later with disease progression. The outcome is much worse in adults than in children.70
Pathology
According to the latest pathologic reports, Rasmussen’s encephalitis is the result of an autoimmune process with astrocytes as the primary target.71 A specific feature is astrocytic apoptosis and subsequent loss of astrocytes. In addition, proliferation of microglia, neuronal degeneration, and marked perivascular lymphocytic infiltration are present.
Imaging
MRI
On T2W sequences, high signal intensity abnormality and volume expansion will be seen in the cortex of the temporal or parietal lobe (Fig. 42-6). In the terminal stage, MRI shows destruction of the involved area with unilateral ventricular enlargement.
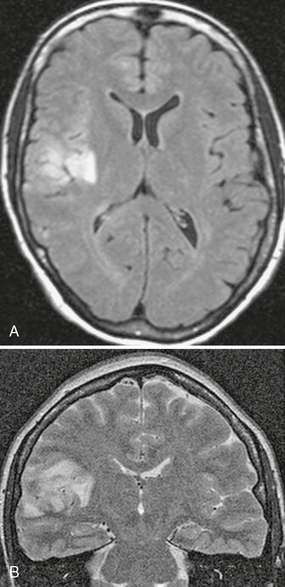
FIGURE 42-6 Suspected Rasmussen’s encephalitis. This 27-year-old woman presented with seizures. Axial FLAIR (A) and coronal T2W (B) MR images show abnormal high signal intensity affecting the cortex and underlying white matter of the right parietal region.
Herpes Simplex Encephalitis
Epidemiology
HSE caused by HSV-1 is the most common cause of sporadic lethal encephalitis, which occurs in about 1 person per 250,000 to 500,000 population per year. In AIDS patients, the estimated frequency of HSV-1 infection is only 2% of autopsy cases.72 In the absence of therapy, mortality exceeds 70% and prognosis is poor because only a minority of individuals return to normal function.
Imaging
CT
CT studies are frequently negative in the early stages of HSE. With disease progression (after 3-5 days), areas of low attenuation may be detected in the temporal lobes.73
MRI
The majority of patients with HSE will show signal abnormalities on scans, but normal MRI results have been reported in approximately 10% of polymerase chain reaction–positive patients who have HSE. On MRI, high signal intensity lesions will be observed on T2W images, with low signal on T1W images (Fig. 42-7). The FLAIR technique has been reported to be even more sensitive than T2W imaging in the detection of abnormalities due to HSV-1 infection, with the earliest imaging findings 48 hours after the onset of symptoms (see Fig. 42-7). Early on, enhancement is usually not apparent and gyriform enhancement on enhanced T1W images can be observed with disease progression (Fig. 42-8).74 T2*W gradient-echo images may show a signal decrease caused by the magnetic susceptibility effect of hemoglobin degradation, suggesting a hemorrhagic component.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree