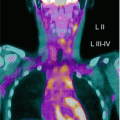
Fig. 17.1
Three-year-old boy with hepatoblastoma. (a) Maximum-intensity projection image and (b) axial images (from left to right: CT, FDG PET, fused FDG PET/CT) show irregular, heterogeneous, and markedly elevated uptake right lobe mass and less uptake in left lobe lesion
17.2 Desmoplastic Small-Round-Cell Tumor
Desmoplastic small-round-cell tumor (DSRCT) is a very rare embryonic neoplasm, primarily affecting adolescent males, that is, considered a type of soft tissue sarcoma. It is aggressive with overall relatively poor prognosis. It often occurs as an abdominal mass, but the disease may involve the peritoneum, pleura, lymph nodes, bones, and other organs [19, 20]. The tumor is composed of small, undifferentiated round or oval hyperchromatic cells with abundant desmoplastic stroma, originated from a primitive pluripotent stem cell with multiphenotypic differentiation and harboring karyotypic abnormality t(11;22) (p13;q12) [21, 22]. Treatment is multidisciplinary that may include debulking surgery, radiation, myeloablative chemotherapy with stem cell rescue, and Y90 microspheres for targeted treatment of liver lesions [23, 24].
Imaging plays a major role in localization and determination of the extent of disease and for evaluating treatment response [25, 26]. Arora et al. evaluated the imaging features of DSRCT in 62 patients [27]. The most common imaging finding was multiple peritoneal soft tissue masses. Metastatic disease was documented in the liver, lungs, spleen, lymph nodes, and bones. FDG PET/CT accurately detected 97.4 % of all lesions. FDG PET/CT has also been reported to be useful in assessment of favorable chemotherapy response with significant decline (by more than 50 %) in metabolic activity of lesions in comparison to pretreatment activity level [28]. A single case report of the usefulness of FDG PET in monitoring of treatment with a mammalian target of rapamycin inhibitor has also been reported [29].
Clinical Case Example
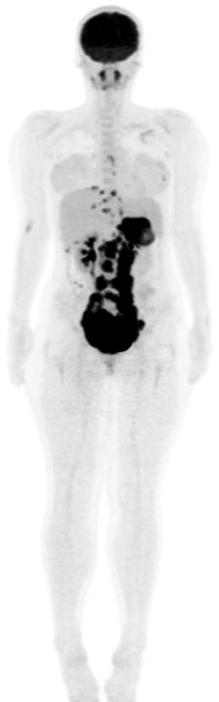
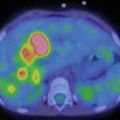
Fig. 17.2
Twenty-year-old woman with DSCRT. Maximum-intensity projection image shows extensive hypermetabolic soft tissue disease involvement
17.3 Langerhans Cell Histiocytosis
Langerhans cell histiocytosis (LCH) is a group of clinically heterogeneous diseases (ranging from indolent involvement of a single organ to a potentially fatal multisystem disease) characterized by accumulation of Langerhans cells (a subtype of dendritic cells of histiocytic origin) of the immune system in various organs [30, 31]. The disease has previously been known as histiocytosis X, eosinophilic granuloma, Letterer-Siwe disease, Hashimoto-Pritzker disease, and Hand-Schüller-Christian syndrome [32]. The current designation of LCH was introduced in 1973 [33]. The disease is now considered neoplastic given that it may be associated with the proto-oncogene BRAF mutation. LCH is a rare clinically unpredictable disease in children (about 3–5 cases per million children younger than 14 years) and can affect many organs including the skin, bone, lymph nodes, liver, lung, spleen, and central nervous system. Bone is the most common site of involvement in 78 % of patients (skull > pelvis > femora > ribs). Skin and lymph node involvement may be present in 50 % and 30 % of patients, respectively [33]. Management of the disease remains controversial but is generally risk adapted [34, 35]. Patients with disease involvement of the skin, bone, and lymph node generally have relatively good prognosis. However, patients with lesions in liver, spleen, lung, central nervous system, or bone marrow are considered in high-risk group and have a worse overall prognosis [36].
Radiography and bone scintigraphy are often employed in the imaging assessment of patients with LCH involvement of the bones [37–39]. Howarth et al. compared bone scintigraphy and skeletal radiographic survey for detection of osseous lesions in 73 patients with LCH [40]. They reported sensitivity and specificity of 100 % and 61 % for skeletal radiographic survey and 91 % and 55 % for bone scintigraphy, respectively. Although fewer bone lesions may be detected on bone scintigraphy in comparison to radiography, however, bone scintigraphy depicts those bone lesions that are metabolically active [41]. Despite the utility of both bone radiography and scintigraphy, both these scans are nonspecific and may not accurately portray the lesion response to treatment. Whole-body MRI has also been employed and in one study was compared to radiography and bone scintigraphy, demonstrating potential advantage of MRI over both these imaging modalities in this clinical setting [42]. Few investigators have also reported that somatostatin receptor scintigraphy with 111In-pentetreotide may be useful [43, 44]. PET-CT with FDG has been shown to be useful for identification of metabolically active lesions, stratification of disease phases, treatment response monitoring and detection of recurrence [45–49]. Kaste et al. evaluated the utility of FDG PET-CT in five patients with LCH at various stages of treatment [50]. The metabolic information was found to be significantly more useful than radiography or bone scintigraphy for assessing disease extent and response to treatment. Mueller and colleagues compared FDG PET and MRI scans in 15 patients with LCH with biopsy or follow-up scans serving as reference standard [51]. The sensitivity and specificity of PET were 67 % and 76 %, respectively. The sensitivity and specificity of MRI were 81 % and 47 %, respectively. The authors concluded that the imaging information provided by PET and MRI is complementary.
Clinical Case Example
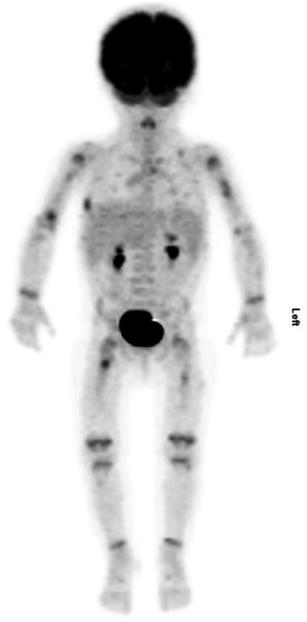
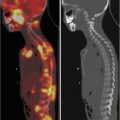
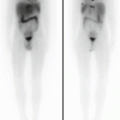
Fig. 17.3
Seven-month-old female with anemia, persistent fevers, and several lytic bone lesion-related LCH. Maximum-intensity projection images show bone lesions in bilateral humeri, a right lateral rib, and bilateral femora
17.4 Adrenocortical Carcinoma
Adrenocortical carcinoma (ACC) is a rare malignancy with an estimated annual incidence in the United States of approximately 1–2 per million (0.02 % of all cancers), a bimodal peak frequency in patients 5 years or younger and ages 30–50 years and overall relatively poor 5-year survival of 20–45 % [52, 53]. A feature of ACC is that it may be endocrinologically active and very aggressive clinically [54, 55]. Surgical resection of the tumor offers the best probability of potential cure.
The primary imaging modalities for initial assessment of ACC are CT and MRI [56]. PET with FDG has also been found to be useful since most ACC tumors are metabolically active [57–59]. In a recent study of 51 patients preoperatively, FDG PET showed high sensitivity and specificity of 95 and 97 % for diagnosis of ACC [60]. Leboulleux and colleagues showed that FDG PET/CT is more sensitive and specific than CT for detection of metastatic disease, 90 % and 93 % for FDG PET/CT and 88 % and 82 % for CT, respectively [61].
Despite the suggested diagnostic utility of FDG PET in ACC, other studies have found that FDG PET may not be useful in the evaluation of the remaining adrenal gland after treatment with mitotane and may not have prognostic utility [62]. Tessonnier et al. in a retrospective study of 37 patients with ACC found no correlation between the primary tumor FDG uptake level and patient outcome as depicted by overall survival and disease-free survival at 2 years [63].
Other PET tracers have also been examined in the imaging evaluation of patients with ACC. 11C-metomidate was performed in 15 patients with adrenal mass and showed the ability to discriminate the cortical from non-cortical lesions [64]. Metomidate binds to CYP11B enzymes of the adrenal cortex [65]. Kreissl and colleagues reported recently that the 123I-label of metomidate (123I-iodometomidate) accumulates in both primary and metastatic lesions of ACC but with overall sensitivity of 38 % (at a specificity of 100 %) [66]. Cytotoxic chemotherapy and mitotane treatment did not affect tracer uptake in the lesions. Preclinical mouse studies of radiolabeled Fab’2 fragment of the anti-adrenocortical Ac5 antibody has also been reported [67].
Clinical Case Example
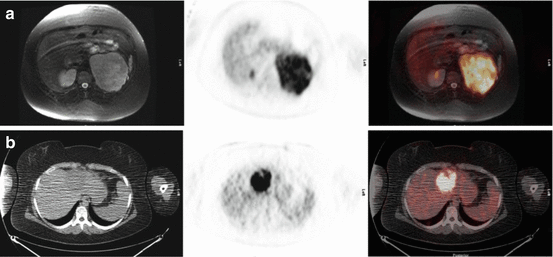
Fig. 17.4
Fifteen-year-old girl with newly diagnosed adrenal mass and liver metastasis related to ACC. (a) Axial images (from left to right: fat-saturated T2-weighted MRI, FDG PET, fused MRI, and FDG PET/CT) show the primary adrenal tumor and (b) axial images (from left to right: CT, FDG PET, fused FDG PET/CT) show the left hepatic lobe metastasis
17.5 Summary
We discussed the clinical demographics and the potential utility of scintigraphy including PET in the imaging evaluation of four uncommon pediatric neoplasms, hepatoblastoma, desmoplastic small-round-cell tumor, Langerhans cell histiocytosis, and adrenocortical tumor. In general, PET appears to have a useful role in diagnostic and prognostic assessment of these rare tumors, although additional prospective studies will be helpful to draw additional experience.
Acknowledgment
National Cancer Institute, National Institutes of Health, grant R01-CA111613 (H. Jadvar)
References
1.
2.
3.
4.
5.
6.
Seitz G, Fuchs J, Schaaefer JF et al (2012) Molecular imaging and photodynamic therapy in hepatoblastoma. Front Biosci (Elite Ed) 4:487–492CrossRef
7.
8.
Dachman AH, Parker RL, Ros PR et al (1987) Hepatoblastoma: radiologic-pathologic correlation in 50 cases. Radiology 164:15–19CrossRefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree