Key Points
- ▪
Review of patient and study issues, planning, and preparation are required before the scan is performed. The degree of planning and preparation has a direct impact on the adequacy of the obtained images.
- ▪
Attention to technical factors and selection of optimal scanning settings is essential to acquiring a complete data set of optimal quality.
- ▪
Following the scan:
- ▪
Review the quality of the study and identify artifacts (some artifacts can be eliminated by changing reconstruction parameters).
- ▪
Select reconstruction parameters and verify that the reconstructions are optimal for the referring question.
- ▪
Review the study to address the cardiac (cardiovascular) indication.
- ▪
Review the study to address noncardiac pathologies.
- ▪
Archive the scan.
- ▪
- ▪
Much of the potential quality of a study can be forfeited by poor preparation; conversely, many motion artifacts, electrocardiographic (ECG) artifacts, contrast artifacts, and field of view (FOV) errors can be avoided by sufficient preparation.
- ▪
Prepare the patient for the study (e.g., describe sensations to expect, explain the need for no movement).
- ▪
Have the patient practice breath-holding until it is being reliably performed.
- ▪
Establish a good ECG signal.
- ▪
Establish reliable IV access in the right arm.
- ▪
Consider β-blocker use and monitor accordingly.
Overview of the Process
Pre-Scanning Issues
Study Issues
- □
Review the case history.
- □
Establish:
- •
The primary indication for the study
- •
Other indications for the study
- •
- •
Contraindications to the study
- •
The appropriateness of CT versus other alternative modalities for the indication and for the patient
- •
- □
Select:
- •
The optimal scanning protocol
- •
The optimal radiation-lowering features
- •
The optimal contrast injection technique
- •
Patient Issues
- □
Appropriate patient exclusion and selection
- □
Appropriate preparation:
- •
To ensure predictable contrast delivery, via the right side:
- •
IV location: right antecubital vein
- •
IV size: sufficient size (≥18G) to allow for the needed high flow rate (4–5 mL/sec)
- •
- •
To avoid problematic increase in heart rate during the study:
- •
Ensure that the patient understands that there will be noise during the scanning.
- •
Confirm that the patient understands that there will be a warm sensation during the study.
- •
Consider β-blocker use; evaluate for risk-benefit ratio.
- •
Short-term oral use
- •
Use with or without IV supplementation
- •
- •
- •
Administer sublingual nitroglycerin for coronary studies.
- •
To achieve predictable position of the heart during the scan:
- •
The patient must understand and practice consistent breath-holding for the study.
- •
- •
To avoid motion artifacts, the patient must understand that he or she must not:
- •
Move during the scan
- •
Take a second breath during the scan
- •
Release the breath during the scan
- •
Swallow during the scan
- •
- •
Scanning Issues to Resolve
- □
Verification of protocol selection:
- •
Decide whether or not to perform:
- •
A noncontrast scan first
- •
Calcium scoring
- •
A delayed scan
- •
- •
Select gating mode:
- •
Prospective gating
- •
Retrospective gating
- •
- •
- □
Verify contrast injection:
- •
Technique
- •
Injection technique:
- •
Single injection
- •
Dual injection
- •
Triple injection
- •
- •
Bolus technique
- •
“Test bolus” method
- •
“Bolus tracking” method
- •
- •
- •
Rate, duration, and amount of injection
- •
Site of injection
- •
- □
Selection of an FOV that is suitable to capture both the superior and inferior extent of the region of interest (e.g., coronary tree). Recall that the breath taken during the study may not be of the same depth as the one taken for the topographic view that the FOV was selected on.
- □
The greater the needed anatomic coverage, the greater the FOV, but the greater the FOV:
- •
The greater the radiation dosage
- •
The larger the voxel size
- •
- □
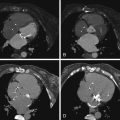
In order of increasing FOV size ( Fig. 2-1 ):
- •
CTA of the coronary tree only
- •
CTA with saphenous vein graft coverage
- •
CTA with internal mammary arterial graft imaging
- •
Pulmonary embolism (PE) protocol
- •
Aorta protocol
- •
Lower extremity vasculature

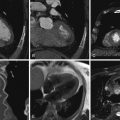
Figure 2-1
Scout images obtained before a CT angiogram. The scout makes it possible to determine the appropriate field of view for the cardiac CT study.
- •
- □
Selection of technical factors appropriate for the patient’s body habitus (e.g., milliamps)
Post-Scanning
Quality Assurance Analysis
- □
Verification that the achieved anatomic coverage is complete:
- •
Did the upper limit of the scan capture the upper extent of anatomic interest?
- •
Did the lower limit of the scan capture the lower extent of anatomic interest?
- •
- □
Verification of quality of the scan
- •
Quality check:
- •
Artifacts
- •
Signal-to-noise ratio (SNR)
- •
- •
Quality assurance (QA) of the ECG:
- •
Minimal heart rate change during the study
- •
No arrhythmias
- •
- •
QA of the contrast technique:
- •
Contrast opacification quality
- •
If coronary CTA:
- •
Left heart chambers and aorta: excellent contrast effect
- •
Right heart: mild residual contrast effect
- •
- •
- •
Image Reconstruction
- □
Reconstruction of images from the data set as needed to address the referring question ( Fig. 2-2 )
- •
Standard: axial, sagittal, coronal
- •
With or without the following views:
- •
Cardiac-specific
- •
3D
- •
Maximum intensity projection
- •
Curved multiplanar reconstruction
- •
Straightened
- •
Cross-sectional perpendicular view to the centerline (intravascular ultrasound view)
- •
Other
- •

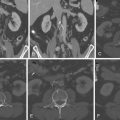
Figure 2-2
Tissue Doppler recording at the mitral annulus level in four different patients, all with corresponding ECG tracings. In each case the motion-free interval, the ideal phase for CCT image acquisition, is indicated on the last cardiac cycle of the tracing by a vertical red line. It can be seen that the motion-free interval is generally very short. The tracings in panel B reveal that lower heart rate is associated with a longer “diastasis” motion-free phase between early and late diastolic filling/motion, which allows for better quality CCT image acquisition.
- •
- □
If there is reason to be concerned that:
- •
Image reconstruction did not depict the heart without motion in diastole:
- •
Reconstruct the images at another phase of the cardiac cycle (e.g., 70%, 75%, 80%)
- •
- •
Image reconstruction did not depict the heart at end-systole:
- •
Reconstruct the images at another phase of the cardiac cycle (e.g., 35%, 40%).
- •
- •
Image Review
- □
Cardiac pathology review
- •
Review axial images
- •
Use additional modalities as suited to the case
- •
- □
Noncardiac pathology review
- □
Comparison to previous studies
- □
Are further reconstructions needed?
Study Archiving
- □
Images to be archived must be selected.
- •
Selected image sequences will be archived; the data set seldom is archived.
- •
- □
Site of archiving must be selected.
Exclusions to CT Angiography
Critical steps to achieve safe studies of optimal quality include:
- □
Correct selection of patients
- □
Correct exclusion of patients who will have poor quality studies
- □
Correct exclusion of patients who will be exposed to higher risk
- □
Best preparation of the patient
- □
Best preparation of the ECG signal
- □
Heavy coronary calcium predictably impairs interpretation of coronary CTA.
- •
Calcium above a certain level observed on a calcium scoring protocol before CTA may preclude the utility of performing the CTA (e.g., Agatston score ≥ 750 or ≥ 1000).
- •
Calcium scoring may reduce overall radiation exposure, if used to select for CTA, which may be the most practical use of calcium scoring.
- •
- □
Temporal resolution issues
- •
Tachycardia
- •
Arrhythmia, if very irregular
- •
Heart rate variability
- •
- □
Contrast dye intolerance
- •
Renal insufficiency (creatinine clearance < 30 mL/min)
- •
Decompensated heart failure
- •
Severe allergy
- •
- □
Dyspnea: i.e., the patient is not expected to be able to breath-hold for 20 to 30 seconds.
- □
Claustrophobia
- □
β-Blocker intolerance (if β-blockers are expected to be needed)
- □
Morbid obesity
- □
Stents (probably). Large stents less so; small stents more so.
- □
Intracardiac devices (e.g., pacemakers or implantable cardioverter defibrillators may limit the evaluation of the right coronary artery)
Optimal Selection and Preparation
The patient must be prepared well for what to expect. Although scanning proceeds automatically once initiated, any movement during the scanning or substantial heart rate acceleration will compromise the quality of the acquired images. Therefore, the patient must completely understand what to do and what not to do. If, for example, a patient is aware of the sensation to expect from dye injection, his or her anxiety and subsequent increase in heart rate during the study will be less.
Patient Review
Patient review is discussed in detail in Abbara et al.
Initial Screening
- □
Initial screening is described in Box 2-1 .
BOX 2-1
- 1.
History taking to evaluate for:
- a.
Pregnancy or potential pregnancy: According to ACR recommendations “All imaging facilities should have policies and procedures to identify pregnant patients prior to imaging, and to consider any possible risks to the fetus of any planned administration of contrast material, taking into consideration the potential clinical benefits of the examination.”
- b.
Contraindication to contrast media or other medications including β-blockers and nitroglycerin
- c.
Renal insufficiency and risk of contrast-induced nephrotoxicity (CIN)
- d.
Prior allergic reactions to any allergens
- e.
Active bronchospastic disease, hypertrophic cardiomyopathy, severe aortic valve stenosis, or other precautions or contraindications to β-blockers
- f.
Current medications (especially sildenafil, vardenafil, tadalafil, or metformin)
- g.
Any other pertinent medical history
- a.
- 2.
Assessment of the ability to follow breath-hold commands and perform inspiratory breath-hold
- 3.
Assessment of body weight
- 4.
Assessment of heart rate (preferably after inspiration) and arrhythmia
- 5.
Assessment of blood pressure
Initial Screening
Reprinted with permission from Abbara S, Arbab-Zadeh A, Callister TQ, et al. SCCT guidelines for performance of coronary computed tomographic angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comp Tomogr. 2009;3:190-204.
- 1.
Pretest Instructions
- □
Pretest instructions are presented in Box 2-2 .
BOX 2-2
- 1.
No food for 3–4 hours before examination.
- 2.
May drink water or clear fluids up until time of examination (patient should be well hydrated for renal protection, for ease of establishing venous access, and to avoid postprocedure hypotension).
- 3.
No caffeine products for 12 hours before examination, because they might hinder efforts to reduce the heart rate before scanning. This includes coffee, tea, energy drinks, energy pills, diet pills, and most soda.
- 4.
Take all regular medications the day of examination, especially blood pressure medicine.
- 5.
Take pre-medications for contrast allergy as prescribed by the ordering physician. As an example, the standard Greenberger regimen is prednisone, 50 mg by mouth, 13, 7, and 1 hour before contrast exposure, in addition to diphenhydramine 50 mg by mouth 1 hour before contrast exposure.
- 6.
Metformin use must be discontinued for at least 48 hours after the contrast administration. Metformin itself is not nephrotoxic, but it is exclusively renally cleared. If renal failure is precipitated by iodinated contrast, a toxic accumulation of metformin may result, which can induce lactic acidosis. There is no evidence that withholding metformin before a contrast procedure is protective, although this approach has been adopted by some.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


- 1.