Chapter 13 Pancreatic Neuroendocrine Tumors
Introduction
A number of neuroendocrine tumors (NETs) exist. This chapter discusses pancreatic neuroendocrine tumors (PNETs) such as insulinomas, gastrinomas, VIPomas (vasoactive intestinal polypeptidomas), glucagonomas, and nonfunctional PNETs. PNETs are a subgroup of gastroenteropancreatic NETs.1 The term neuroendocrine is derived from the similarity to neural cells in the expression of proteins such as synaptophysin, neuron-specific enolase, and chromogranin A.2 Previously, PNETs were often referred to as “carcinoid tumor” or “islet cell tumor.” However, with the World Health Organization (WHO) classification of 2000,2 these have been replaced by the more generic term pancreatic neuroendocrine tumor (PNET). The WHO classification reserves the term carcinoid for well-differentiated NETs of purely gastroenteric origin.
PNETs are thought to arise from a common precursor neuroendocrine cell that shares features with similar such cells throughout the body constituting the “neuroendocrine system” of the body.3 The embryologic origin of this common precursor cell is controversial. Masson and coworkers4 suggested that the neuroendocrine cell was an endocrine cell derived from the gastrointestinal epithelium, suggesting an endodermal origin. However, in 1968, Pearse5 showed that all the neuroendocrine cells throughout the body shared various features including the amine precursor uptake and decarboxylation (APUD) capacity and postulated that the neural crest was the common origin for these cells called the APUD cells. These cells have pluripotential capabilities including differentiating into various types of NETs.6 More recently, an endodermal origin to the pluripotent cells has been favored.7
Epidemiology and Risk Factors
The data on the incidence of PNETs in autopsy series are limited and varied. PNETs have been reported to occur in between 2% of autopsies8 and 10% in another autopsy series.9 However, the annual incidence is reported to be only between 2.5 and 5 per 100,000 per year, suggesting that this apparent lower incidence may be related to the detection of predominantly functional tumors.10 The incidence of PNETs is increasing.11 These still represent a small percentage of pancreatic tumors, accounting for 1% to 2% of all primary pancreatic tumors.9 Recent literature indicates that the majority of PNETs are actually nonfunctional, ranging between 50% and 80% of all PNETs.12–14 Overall, PNETs have a better long-term survival and overall prognosis when compared with ductal adenocarcinoma of the pancreas. The 5-year survival for PNETs ranges from 30% in nonfunctional PNETs to 97% in a subgroup of functional PNETs, the insulinoma.15
Four syndromes have been associated with a higher incidence of PNETs. These are the multiple endocrine neoplasia type I (MEN I or Wermer’s syndrome); von Hippel–Lindau (vHL) syndrome; neurofibromatosis-1 (NF-1 or von Recklinghausen’s syndrome), and tuberous sclerosis (TS).16 The relative frequency with which patients with these disorders develop PNETs is MEN I > vHL > NF-1 > TS.
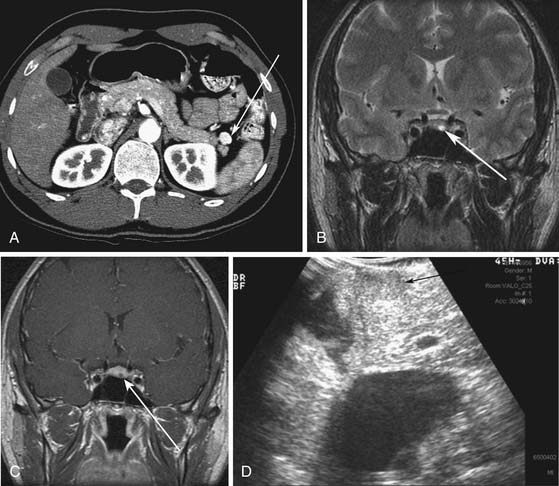
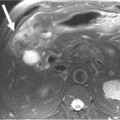
MEN I is a syndrome characterized by tumors/hyperplasia of endocrine glands. This is inherited as an autosomal dominant trait. MEN I gene mutations can be identified in 78% to 93% of MEN I patients.17 MEN I patients usually have a family history of MEN I. The syndrome is characterized primarily by tumors of the parathyroid glands, endocrine components of the gastrointestinal and pancreatic systems, and the pituitary gland (Figure 13-1). The different incidences of PNETs in patients with MEN I are nonfunctional tumors (80-100%), gastrinomas (20-61%), insulinomas (7-31%), and other functional PNETs (<5%). With increasing control of symptoms of excess hormone production, PNETs have become an important factor in determining survival in patients with MEN I. Patients with MEN I syndrome have a higher incidence of premature death and PNETs have been attributed to be one of the causes for this.18,19 Thus, identifying and treating PNETs in this subgroup of patients may have a significant impact on survival.
vHL is a syndrome characterized by autosomal dominant inheritance. vHL patients develop hemangioblastomas of the central nervous system (prevalence 44-72%) including retinal hemangioblastomas and cerebellar hemangioblastomas, renal cysts and renal cell carcinomas (25-60%), endolymphatic sac tumors (10%), and pheochromocytomas (10-20%).20 Pancreatic tumors or cysts develop in 35% to 70% of patients (Figure 13-2). Pancreatic cysts or serous cystadenomas develop in 17% to 56% of patients and PNETs occur in 8% to 17% of patients.21 The majority of the PNETs are nonfunctional.
NF-1 is more common than MEN I and vHL syndrome. Duodenal somatostatinomas (0-10%) and ampullary carcinoid tumors can occur in NF-1 patients. Pancreatic somatostatinomas (functional PNET) occur in patients with NF-1 at a much lower prevalence than the duodenal somatostatinoma.22 Insulinomas and nonfunctional PNETs are less common but can also be present in patients with NF-1.23
TS also has autosomal dominant inheritance. Patients with TS have been reported to have both functional and nonfunctional PNETs (<1%) especially patients with the TSC-2 gene mutation.16
Anatomy and Pathology
Anatomy
The pancreas is an elongated organ varying from 12.5 to 15 cm with a weight from 60 to 100 g. It is composed of both exocrine portions and endocrine portions. Multiple fat lobulations are seen on the surface due to invaginating adipose tissue.24 The portion of the pancreas located to the right side of the abdomen is recognized as the head and lies within the curve of the duodenum. A conical elongation of the pancreas extending posterior to the superior mesenteric vessels is the uncinate process. A narrow neck, anterior to the superior mesenteric vein (SMV), connects the head to an elongated body that tapers in the left upper quadrant to form the tail. The pancreas is located in the retroperitoneum except for a portion of the tail of the pancreas, which may be intraperitoneal along the gastrosplenic ligament.
The arterial supply for the pancreas is from the pancreaticoduodenal arteries and the splenic artery. The celiac trunk gives rise to the common hepatic artery, splenic artery, and the left gastric artery. The common hepatic artery gives off the gastroduodenal artery (GDA) and becomes the proper hepatic artery. The GDA divides into the superior pancreaticoduodenal arteries (anterior and posterior) and the right gastroepiploic artery. The anterior and posterior superior pancreaticoduodenal arteries supply the cranial aspect of the pancreas and descend between the duodenum and the pancreas, supplying both these organs. These arteries anastomose with the anterior and posterior inferior pancreaticoduodenal arteries and the pancreatic branches of the splenic artery. The anterior and posterior inferior pancreaticoduodenal arteries are branches of the inferior pancreaticoduodenal artery arising from the superior mesenteric artery (SMA). The splenic artery gives rise to numerous small branches that supply the body and tail of the pancreas. One branch, the pancreatica magna artery, runs along the body of the pancreas. The dorsal pancreatic artery is another branch usually of the splenic artery but can have a variable origin. It is usually located posterior to the portal venous confluence, passing behind the splenic vein.25 This artery divides into two terminal bifurcating branches.
The venous drainage of the pancreas parallels the arterial supply. Four major pancreaticoduodenal veins drain the pancreas in addition to multiple smaller pancreatic veins that drain the pancreas and enter the splenic vein directly.26 The inferior pancreaticoduodenal veins drain the inferior portion of the pancreas and enter the first jejunal vein, which drains into the SMV. The posterior superior pancreaticoduodenal vein drains directly into the caudal portion of the portal vein. The anterior superior pancreaticoduodenal vein runs horizontally and drains into either the gastrocolic branch of the SMV or the right gastroepiploic vein, which drains into the SMV. The SMV and the splenic veins join posterior to the neck of the pancreas to form the portal vein. The inferior mesenteric vein may enter at this confluence in one third of patients, enter the splenic vein close to this confluence in one third of patients, or enter the SMV in one third of patients.
Tumor Types
PNETs can be divided into the functional and nonfunctional. The main functional PNETs include insulinomas, gastrinomas, glucagonomas, somatostatinomas, VIPomas, growth hormone releasing factor (GRFomas). The nonfunctional PNETs are named as such owing to either lack of hormone production by the tumor or lack of clinically identifiable symptoms related to the tumor, as shown in Table 13-1.
| GRADE | MITOSES | KI-67 INDEX |
|---|---|---|
| 1 | <2 | ≤2% |
| 2 | 2-20 | 3-20% |
| 3 | >20 | >20% |
PNETs are typically well-circumscribed solitary masses within the pancreas.27 They can vary in size from less than 1 cm to large tumors greater than 15 cm. They can be white to yellow or pink-brown in color.
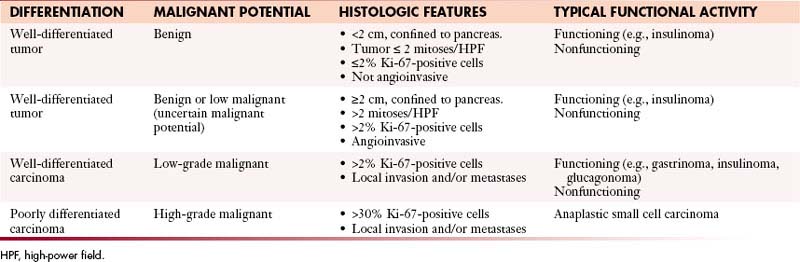
The WHO classification has differentiated PNETs into well-differentiated tumors with benign, benign or low malignant potential; well-differentiated carcinomas with low-grade malignant potential with local invasion or metastases; and poorly differentiated carcinomas with high-grade malignant potential (Table 13-2).28 Well-differentiated tumors or carcinomas are characterized by monomorphic tumor cells with abundant cytoplasm and low mitotic index. They tend to have a well-developed stroma with well-formed blood vessels and may exhibit a trabecular, glandular, or acinar pattern. Poorly differentiated carcinomas are characterized by solid appearance with large areas of necrosis and cells demonstrating a round small tumor cell pattern with high mitotic index and cellular atypia.29
Rindi and coworkers30 and the ENETS (European Neuroendocrine Tumor Society) consensus statement address the issue of tumor grading. The proliferative activity of tumor cells can be assessed by counting mitoses or by immunostaining for Ki-67 (using the MIB1 antibody), which is a cell-cycle–dependent marker as shown in Table 13-1. In a spot where there is high mitotic density, mitoses can be counted on 10 high-power fields (2-mm2 area) at 40 times the magnification and the Ki-67 index can be determined by assessing 2000 tumor cells in an area of high nuclear labeling.
Clinical Presentation
The clinical presentation of PNETs is variable depending on whether these tumors are functional or nonfunctional as shown in Table 13-2. Functional tumors including insulinomas, gastrinomas, glucagonomas, and VIPomas are discussed here.
Patterns of Tumor Spread
The important factors to be defined in a patient with a new diagnosis of PNET are
1. Whether the tumor is functional or not.
2. If the tumor is functional, which subtype does it belong to?
These factors will help in predicting the pattern of spread.
Staging
The WHO classification has differentiated PNETs into well-differentiated tumors with benign, benign or low malignant potential that are confined to the pancreas28; well-differentiated carcinomas with low-grade malignant potential with local invasion or metastases; and poorly differentiated carcinomas with high-grade malignant potential with local invasion or metastases (see Table 13-2).
The ENETS met to define further standards in stratification of the gastroenteropancreatic NETs and proposed tumor-node-metastasis (TNM) staging for different NETs.30 These guidelines were evaluated for consensus among the 62 experts from different countries.
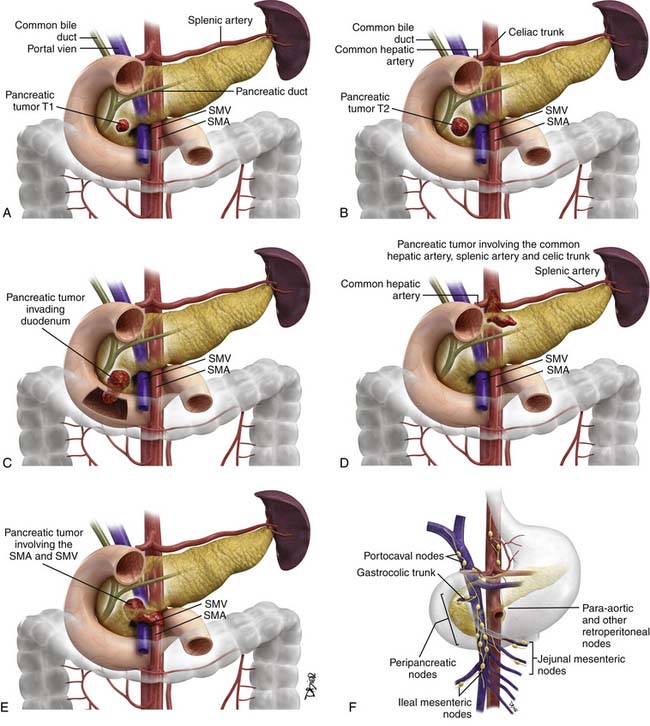
The proposed TNM staging for PNETs is shown in Table 13-3 and Figure 13-3.
Table 13-3 Proposed Tumor-Node-Metastasis Staging
| DESCRIPTION | |
|---|---|
| T Stage | |
| Tx | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| T1 | Primary tumor limited to the pancreas and < 2 cm in size |
| T2 | Primary tumor limited to the pancreas and 2-4 cm in size |
| T3 | Primary tumor limited to the pancreas and > 4 cm in size OR primary tumor is invading the duodenum or bile duct |
| T4 | Primary tumor is invading adjacent organs or the walls of large vessels such as the celiac trunk or superior mesenteric artery |
| N Stage | |
| Nx | Regional lymph nodes cannot be assessed |
| N0 | Regional nodes not involved |
| N1 | Regional nodes involved |
| M Stage | |
| Mx | Distant metastases cannot be assessed |
| M0 | No distant metastases |
| M1 | Distant metastases |
| Proposed Group Stages | |
| STAGE | TNM |
| I | T1N0M0 |
| IIa | T2N0M0 |
| IIb | T3N0M0 |
| IIIa | T4N0M0 |
| IIIb | Any T N1M0 |
| IV | Any T Any N M1 |
Key Points Staging
Grouped Staging
| Stage I | T1N0M0 |
| Stage IIa | T2N0M0 |
| Stage IIb | T3N0M0 |
| Stage IIIa | T4N0M0 |
Imaging
Ultrasound
Ultrasound is a noninvasive and widely available modality that may be the first imaging tool used especially in evaluation of patients with nonfunctional PNETs who present with symptoms related to mass effect. Owing to operator variability and patient variability, a wide range of sensitivities and specificities have been reported. According to a recent consensus statement, the mean detection rate of the primary tumor by ultrasound is 39%, ranging from 17% to 79%.31
For optimal detection of PNETs, the patient should be asked to drink water before the ultrasound to provide a good window through the stomach for evaluation of the pancreas. The patient can be positioned in the supine, left lateral decubitus, or standing position. The PNET is typically a well-defined hypoechoic mass when compared with the rest of the pancreas. They tend to be vascular on Doppler imaging and may demonstrate a hyperechoic halo. Tumors at the edge of the pancreas or located close to the duodenal wall may not be well visualized.32 Intraoperative ultrasound has a higher detection rate with a mean detection rate of 92% (range 74-96%) according to the consensus statement.31
For the detection of lymph node metastases, ultrasound showed a detection rate of 18% according to one study.33 For the detection of liver metastases, one study showed that ultrasound had a sensitivity of 88% and a specificity of 95%.34 Liver metastases may be hypoechoic when small (<1 cm) and tend to be hyperechoic as they get larger. Involvement of the common bile duct, pancreatic duct, and vessel can be assessed well in patients by ultrasound. Patients with large body habitus are not good candidates for sonographic evaluation and should be referred to computed tomography (CT) or magnetic resonance imaging (MRI) owing to poor penetrability by ultrasound.
Endoscopic Ultrasound
A mean detection rate of 90% (range 77-100%) was shown in 10 studies according to the consensus statement.31 EUS also had a mean detection rate of 92% (range 88-94%) in the detection of insulinomas alone. The insulinomas can be difficult to diagnose on cross-sectional imaging owing to their size and may not be well seen on somatostatin (SST) receptor scanning because of small size or a lack of SST receptor subtypes that bind the radiolabeled octreotide with high affinity. EUS has been shown to have a role in patients with MEN I and also vHL in which the PNETs may be less than 1 cm and can be difficult to detect by other imaging modalities.20,35–37 EUS can be used along with fine-needle aspiration (FNA) to obtain tissue samples and confirm the diagnosis. However, EUS is not widely available and is operator-dependent. The entire liver and portions of the tail of the pancreas may not be well evaluated by EUS alone. The role of EUS is complementary to that of other cross-sectional imaging in evaluation of PNETs.
Computed Tomography
Multidetector row computed tomography (MDCT) is available in most radiology practices and has a key role in the evaluation of patients with PNETs.
Imaging
The consensus statement reports a mean sensitivity and detection rate of 73% (range for sensitivity 63-82%) with a higher specificity of 96% for PNETs based on CT.31 Liver metastases can be evaluated with a sensitivity of 82% and a mean specificity of 92%.
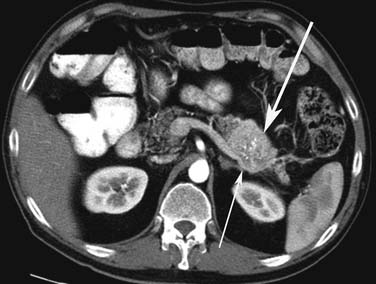
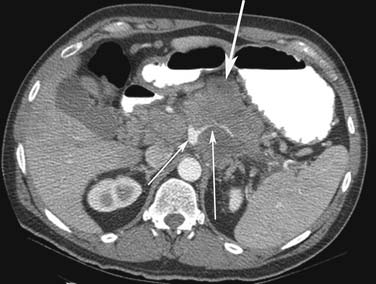
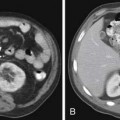
Small PNETs (usually functional tumors) tend to be uniformly hypervascular lesions enhancing in the early phase of enhancement.38 In patients with MEN I syndrome, they are multiple in number and the pancreas should be carefully assessed to localize all the tumors present. They usually tend to be hypervascular to the rest of the pancreas on the portal venous phase of enhancement. Larger PNETs tend to have heterogeneous enhancement. Some of the PNETs may show central areas of necrosis or calcifications (Figure 13-4). Tumor enhancement has been shown to be related to microvascular density according to one study.39 The relationship of these tumors to the surrounding vasculature should be carefully assessed to conform with the proposed TNM staging. Presence of celiac or SMA involvement can preclude surgery (Figure 13-5). The management of venous involvement with narrowing or with tumor thrombus also needs to be addressed preoperatively, especially as related to the technical aspect of venous resection and reconstruction (Figure 13-6). Primary tumors can be hypovascular in up to 40% of cases. Less commonly, primary tumors can be cystic (5-17%).
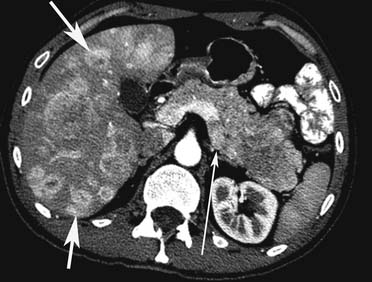
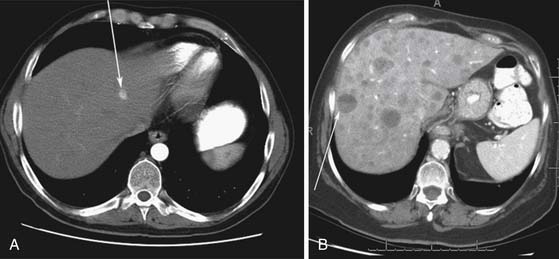
Liver metastases can have a variable appearance. In hypervascular PNETs, the liver metastases are typically hypervascular and are seen in the arterial phase of images well (Figure 13-7A). However, like the primary tumors that are hypovascular, the liver metastases are frequently hypovascular and are seen better in the portal venous phase of enhancement. These metastases may have areas of central necrosis. Some cystic primary tumors can have cystic liver metastases with a fluid-fluid level (see Figure 13-7B).