Part 11 Tumor (not PET)
Case 125
Clinical History
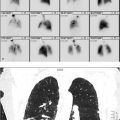
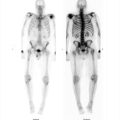
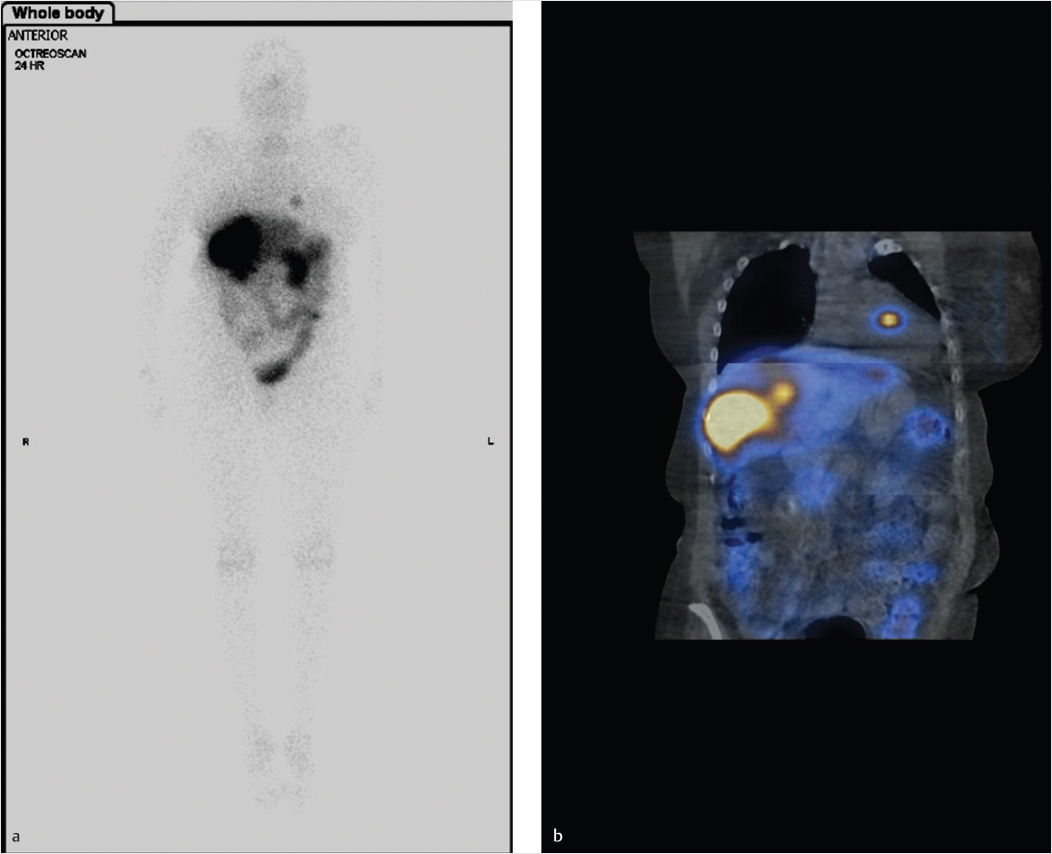
69-year-old female with history of metastatic carcinoid and a cardiac mass initially noted on echocardiogram (▶Fig. 125.1).
Key Finding
Abnormal uptake on In-111 pentetreotide scan (octreoscan)
Top 3 Differential Diagnoses
Neuroendocrine tumor. In-111 has a physical half-life of 67.3 hours and gamma emission peaks of 173 and 247 keV. When coupled with pentetreotide, a somatostatin analogue, it is used in the evaluation of neuroendocrine tumors. Normal biodistribution includes spleen uptake, followed by the kidneys, bladder, and liver. Physiologic uptake is also normally seen in the blood pool, thyroid gland, gallbladder, and, on delayed images, the bowel. Imaging is performed at 4, 24, and possibly 48 hours, if needed. Whole-body planar imaging is performed with single-photon emission computed tomography (SPECT) or SPECT/CT added to improve sensitivity, especially in the chest and abdomen. Neuroendocrine tumors are derived from amine precursor uptake and decarboxylation (APUD) system cells. Carcinoid and pancreatic islet cell tumors are the most common indications for In-111 pentetreotide scanning. Other neuroendocrine tumors include medullary carcinoma of the thyroid, pheochromocytoma, neuroblastoma, paraganglioma, and small cell lung carcinoma. Octreoscan uptake varies by tumor and its subtypes. For islet cell tumors, octreoscan is most sensitive for gastrinomas and least sensitive for insulinomas.
Non-neuroendocrine tumor. These tumors can also contain somatostatin receptors and, therefore, demonstrate radiotracer uptake on In-111 pentetreotide scan. These most commonly include lymphoma, breast carcinoma, non-small cell lung carcinoma, renal cell carcinoma, meningioma, and gliomas. Radiotracer uptake in these tumors tends to be highly variable. In-111 pentetreotide uptake can also be seen in thymoma and thymic carcinoma, helping to differentiate from thymic hyperplasia. In-111 pentetreotide scan may be ordered to evaluate known thymoma to determine if somatostatin therapy might be effective.
Inflammation. Radiotracer uptake may also be demonstrated with inflammation, typically with chronic inflammatory conditions. This is classically described in granulomatous diseases such as sarcoidosis and tuberculosis. Other chronic inflammatory diseases include rheumatoid arthritis and inflammatory bowel disease. Uptake is secondary to lymphocyte somatostatin receptor expression. It is important to be aware of the patient’s medical history, as these conditions can result in false-positive results.
Diagnosis
Carcinoid metastases in the liver and heart.
✓ Pearls
Physiologic octreoscan uptake: spleen, kidneys/bladder, liver, gallbladder, bowel, and thyroid.
Carcinoid and islet cell tumors are the most common indications for In-111 pentetreotide scan.
Several non-neuroendocrine tumors contain somatostatin receptors and demonstrate uptake.
Uptake can be seen in chronic inflammatory conditions, classically with granulomatous diseases.
Suggested Readings
Intenzo CM, Jabbour S, Lin HC, et al. Scintigraphic imaging of body neuroendocrine tumors. Radiographics. 2007; 27(5):1355–1369 Kvols LK, Brown ML, O’Connor MK, et al. Evaluation of a radiolabeled somatostatin analog (I-123 octreotide) in the detection and localization of carcinoid and islet cell tumors. Radiology. 1993; 187(1):129–133 Tan EH, Tan CH. Imaging of gastroenteropancreatic neuroendocrine tumors. World J Clin Oncol. 2011; 2(1):28–43Case 126
Clinical History
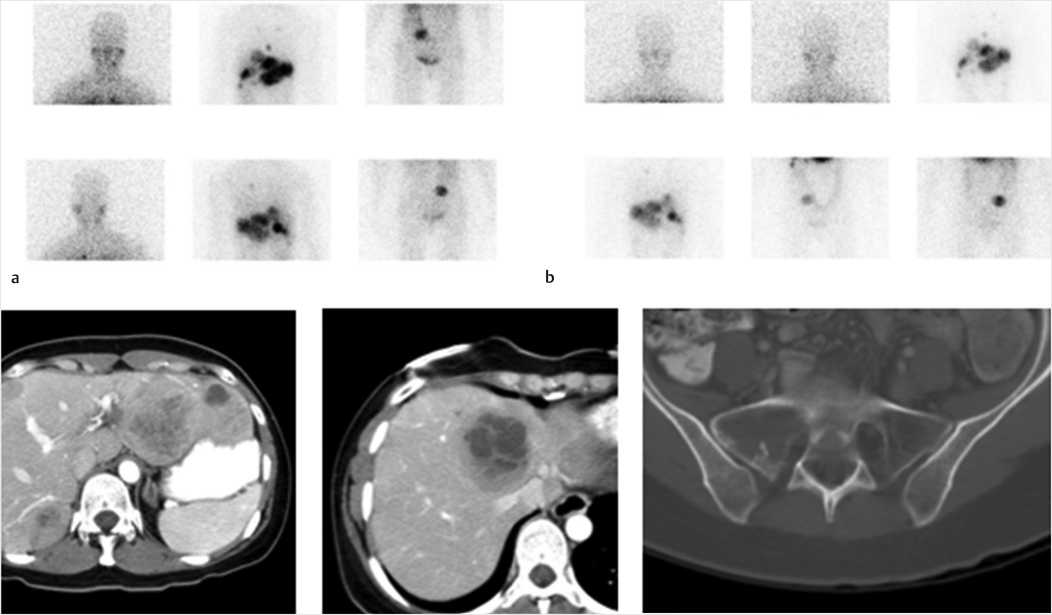
43-year-old male with previous diagnosis of pheochromocytoma, status post resection of the primary lesion and chemotherapy (▶Fig. 126.1). (Case courtesy of Joseph S. Fotos, M.D., Penn State Hershey Medical Center.)
Key Finding
Abnormal uptake on iodine-123 (I-123) metaiodobenzylguanidine (MIBG) scan in an adult
Top 3 Differential Diagnoses
Pheochromocytoma/paraganglioma. I-123 has a half-life of 13 hours and a photopeak of 159 keV. When coupled to MIBG, a norepinephrine analogue, it is useful for imaging sympathetic adrenergic tissue. Normal distribution includes the salivary glands, heart, liver, bladder, and faint kidney uptake. Thyroid uptake is blocked by preadministration of a potassium iodine (Lugol’s) solution. Pheochromocytoma is a catecholamine-producing tumor that arises from the chromaffin cells of the adrenal medulla. The same tumor in an extra-adrenal location is known as a paraganglioma. Pheochromocytomas are classically solid hypervascular masses with high T2 signal intensity on magnetic resonance imaging (MRI). MIBG is most often used to identify clinically suspected tumors, for confirmation of suspected tumor found on anatomic imaging, or to evaluate for metastases. Diagnosis is critical, as catecholamine release may result in life-threatening hypertension or cardiac arrhythmia.
Carcinoid tumor. These tumors arise from endocrine amine precursor uptake and decarboxylation (APUD) cells with about two-thirds of cases originating in the gastrointestinal (GI) tract, with the distal ileum being the most common location, and a quarter of cases arising from the tracheobronchial tree. GI carcinoids may produce 5-hydroxyindoleacetic acid (5-HIAA). They are usually sporadic but are also associated with multiple endocrine neoplasia (MEN) type 1, Zollinger–Ellison syndrome, and neurofibromatosis type I. Lesions may be found incidentally, or may present with bowel obstruction or GI bleeding. Carcinoid syndrome is typically seen when liver metastases are present. Desmoplastic reaction with calcification is classically present with mesenteric involvement, on anatomic imaging. Carcinoid tumors demonstrate variable uptake of MIBG, with the variability not completely understood. However, MIBG appears to be more sensitive for carcinoid tumor in the setting of elevated serum serotonin levels. Octreoscan is more frequently used for carcinoid imaging.
Medullary thyroid cancer. It is a rare neuroendocrine tumor that arises from the parafollicular C cells of the thyroid gland and has a poorer prognosis than papillary and follicular subtypes. The average age of diagnosis is in the sixth decade of life, earlier if associated with MEN type 2 syndrome. Around a quarter of tumors less than 1 cm demonstrate nodal metastases, which jumps to 90% at greater than 4 cm. Typical thyroid imaging radiotracers are of little value, as these tumors poorly concentrate iodine. Fluorine-18 fluorodeoxyglucose positron emission tomography (F-18 FDG PET) is a more useful imaging radiotracer with a sensitivity around 60 to 95%. I-123 MIBG has a reported sensitivity of around 30%. Calcitonin and carcinoembryonic antigen (CEA) are useful biomarkers that aid in diagnosis and follow-up.
Diagnosis
Metastatic pheochromocytoma.
✓ Pearls
I-123 MIBG is favored over octreoscan for pheochromocytoma as there is less physiologic renal uptake.
The distal ilium is the most common location for carcinoid tumor.
Poor iodine concentration in medullary thyroid cancer renders I-123/I-131 imaging of little value.
Suggested Readings
Ganeshan D, Bhosale P, Yang T, Kundra V. Imaging features of carcinoid tumors of the gastrointestinal tract. AJR Am J Roentgenol. 2013; 201(4):773–786 Ganeshan D, Paulson E, Duran C, Cabanillas ME, Busaidy NL, Charnsangavej C. Current update on medullary thyroid carcinoma. AJR Am J Roentgenol. 2013; 201(6):W867–76 Wiseman GA, Pacak K, O’Dorisio MS, et al. Usefulness of 123I-MIBG scintigraphy in the evaluation of patients with known or suspected primary or metastatic pheochromocytoma or paraganglioma: results from a prospective multicenter trial. J Nucl Med. 2009; 50(9):1448–1454Case 127
Clinical History
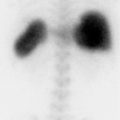
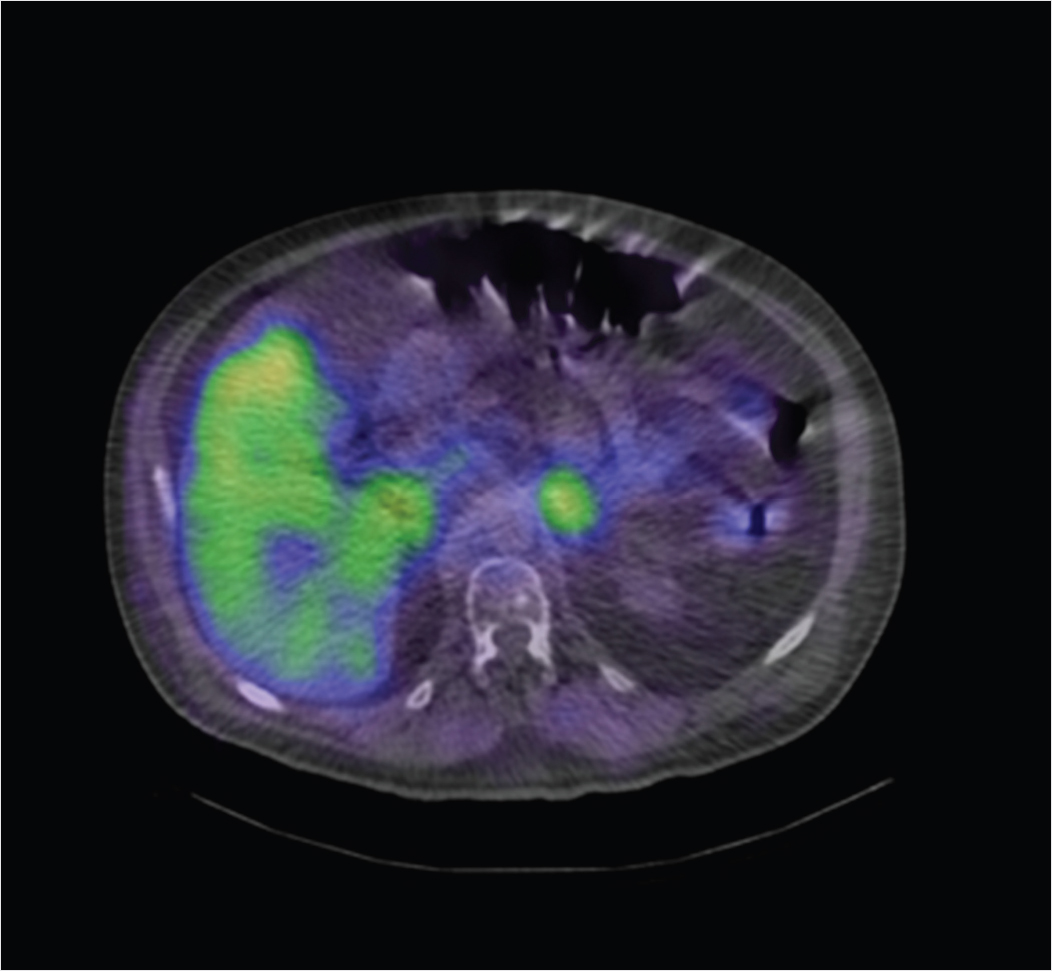
69-year-old male with metastatic paraganglioma referred for evaluation due to an enlarging left supraclavicular nodal mass and increasing pulmonary nodules (▶Fig. 127.1).
Key Finding
I-123 metaiodobenzylguanidine (MIBG) adrenal gland uptake
Top 3 Differential Diagnoses
Normal. MIBG is a norepinephrine analogue that is taken up by norepinephrine transporters and then stored intracellularly in neurosecretory granules. I-123 MIBG is used primarily for evaluation for pheochromocytoma due to less renal uptake than indium-111 (In-111) pentetreotide (which is also highly sensitive for paragangliomas). While norepinephrine transporter is predominantly present in neurons and sympathetic nerves, it is also present in chromaffin cells in the adrenal medulla. This means there is low level normal physiologic uptake of I-123 MIBG in the adrenal medulla, which may either mimic pathologic uptake or hide a small lesion. In general, physiologic adrenal uptake should be homogeneous and less intense than the liver. Physiologic uptake can be asymmetric around half of the time.
Pheochromocytoma. A paraganglioma located within the adrenal gland is known as a pheochromocytoma. This is a usually benign tumor that can episodically secrete catecholamines resulting in symptoms of increased autonomic nervous system stimulation: paroxysmal hypertension, headache, sweating, palpitations, weight loss, etc. I-123 MIBG scintigraphy is used to verify the presence of a paraganglioma in the setting of suspicious clinical symptoms, or to confirm the diagnosis with a known mass and concerning biochemical features. The “rule of 10” has been used for paragangliomas: 10% are extra-adrenal, 10% are familial, 10% are malignant, 10% in pediatric patients, although this may no longer be accurate. Pheochromocytoma is associated with neurofibromatosis type I, von Hippel–Lindau syndrome, multiple endocrine neoplasia (MEN) type IIa and IIb, and Carney syndrome. I-123 MIBG scintigraphy has very high sensitivity for pheochromocytoma, higher than for extra-adrenal paraganglioma, with small lesions being the main cause for a false negative due to low level normal adrenal medullary uptake, as discussed above. Most pheochromocytomas will show focal increased I-123 MIBG activity with intensity exceeding the liver.
Medullary hyperplasia. The amount of I-123 MIBG uptake seen with medullary hyperplasia is variable, but in general this will present with bilateral increased radiotracer uptake with preservation of an adreniform shape.
Diagnosis
Physiologic adrenal gland MIBG uptake. Multiple subsequent CT scans showed continued normal adrenal morphology.
✓ Pearls
Adrenal scintigraphy with I-123 MIBG is useful in cases of suspected pheochromocytoma.
Low-level physiologic adrenal medullary uptake of I-123 MIBG can mask small tumors.
Symmetric homogeneous increased adrenal uptake with normal gland shape suggest hyperplasia.
Pheochromocytoma usually show intense increased I-123 MIBG uptake, greater than liver.
Suggested Readings
Kurtaran A, Traub T, Shapiro B. Scintigraphic imaging of the adrenal glands. Eur J Radiol. 2002; 41(2):123–130 Wiseman GA, Pacak K, O’Dorisio MS, et al. Usefulness of 123I-MIBG scintigraphy in the evaluation of patients with known or suspected primary or metastatic pheochromocytoma or paraganglioma: results from a prospective multicenter trial. J Nucl Med. 2009; 50(9):1448–1454Case 128
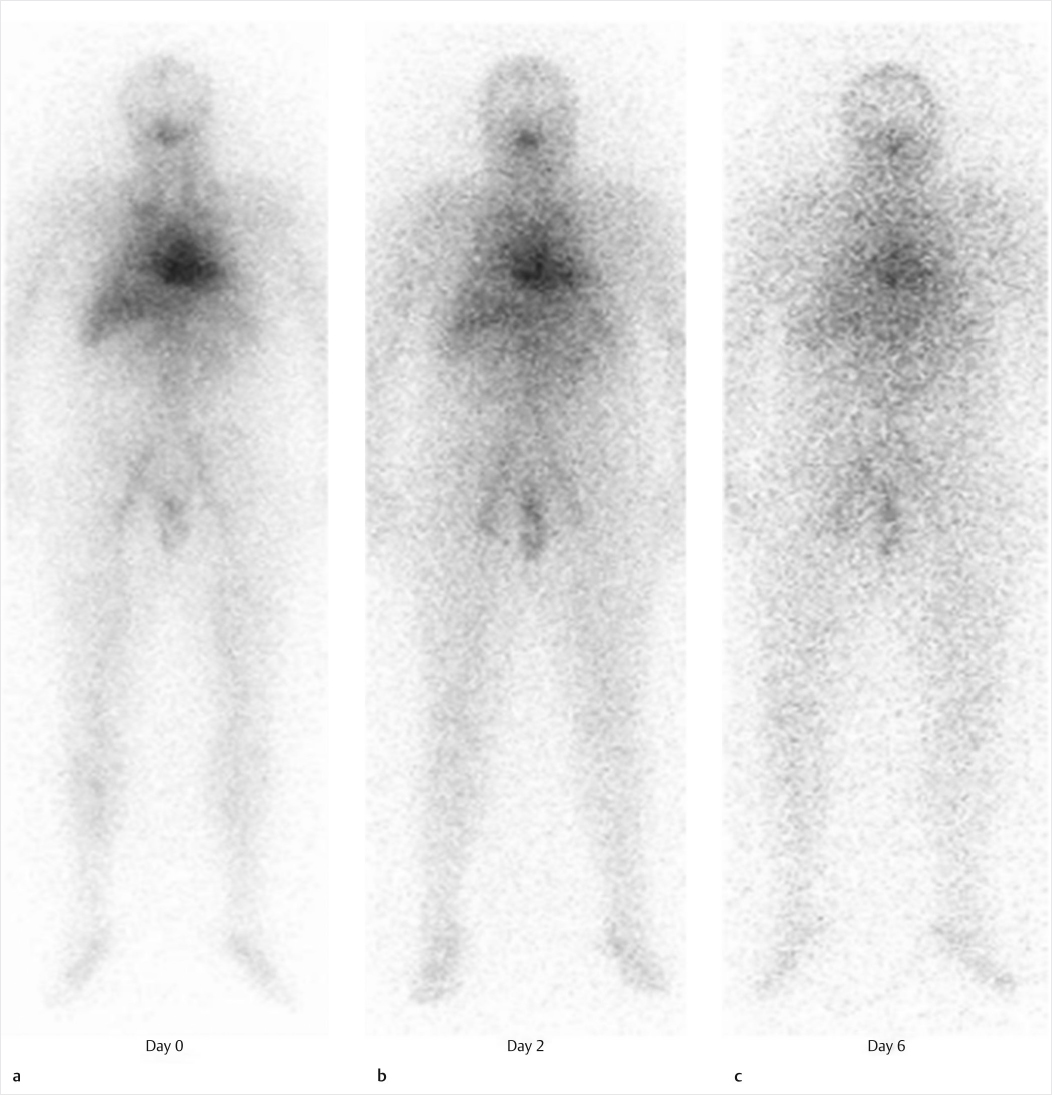
Clinical History
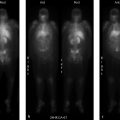
A 54-year-old woman with a relapsed low-grade lymphoma presents for therapy (▶Fig. 128.1).
Key Finding
Radionuclide therapy for lymphoma
Top 2 Differential Diagnoses
Yttrium-90 ibritumomab tiuxetan (Zevalin). Lymphoma, a malignant lymphoid tissue tumor, is generally classified as either Hodgkin’s disease (HD) or non-Hodgkin’s lymphoma (NHL), based on histopathology. NHL can be either of B-cell or T-cell lineage. B cells have specific surface antigens, including CD20 which appears on maturing B cells prior to release from bone marrow. This antigen is the foundation for radionuclide lymphoma therapy. Monoclonal antibodies have been created which target CD20. In the case of Zevalin, the monoclonal antibody is ibritumomab. Standard nomenclature of monoclonal antibodies indicates that ibritumomab is a murine monoclonal antibody (mo = mouse, mab = monoclonal antibody) directed against a tumor (tu = tumor). Tiuxetan is used as a chelator to bind ibritumomab to In-111 for imaging, or Y-90 for therapy. Zevalin was initially approved for the treatment of refractory, relapsed, or transformed CD20 + NHL, but is now authorized as a first-line therapy. Zevalin protocol originally consisted of an initial dose of unlabeled rituximab, followed by 5 mCi of In-111 Zevalin for biodistribution scans obtained on day 1 and day 2, 3, or 4, then an additional dose of unlabeled rituximab prior to the Y-90 Zevalin therapy dose 7 to 9 days later. The biodistribution scan is no longer required. Dosing for the therapeutic infusion is based on weight and platelet count, 0.4 mCi/kg if platelets are over 150,000/mm3, and 0.3 mCi/kg if platelet count is 100,000 to 150,000/mm3. The radiotherapy is contraindicated if initial platelet count is below 100,000 mm3, neutrophil count is below 1500/mm3, and the patient has received external beam radiation to more than 25% of the marrow, or there is history of hypersensitivity to murine antibodies. The purpose of the unlabeled rituximab injections prior to either diagnostic or therapeutic Zevalin infusion is to block CD20 sites present on circulating B cells in the blood pool and spleen, allowing for more circulating time of labeled antibodies and thus more tumoral uptake. It is unclear why, but the unlabeled antibody does not seem to block the CD20 on tumor cells allowing pretreatment to result in increased target uptake. Since the monoclonal antibodies are murine, some patients will develop human antimurine antibody (HAMA). HAMA can make subsequent antibody infusions ineffective as well as initiate an immunologic response which can include fatal anaphylaxis. Because of this, patients must be monitored closely.
Iodine-131 tositumomab (Bexxar). Similar to Zevalin, Bexxar was another approved radiolabeled monoclonal antibody for CD20 + NHL therapy, but is no longer available.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree