Part 4 Lung
Case 36

Clinical History
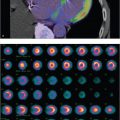
91-year-old male with shortness of breath referred for evaluation for pulmonary embolism (▶Fig. 36.1).
Key Finding
Segmental defect on pulmonary perfusion scan
Top 3 Differential Diagnoses
PE. The clinical diagnosis of PE is difficult as the classic presentation is rare. Untreated PE has high mortality, but anticoagulation therapy has inherent risks. The nuclear medicine evaluation for PE is a pulmonary ventilation (V) and perfusion (Q) scan. This involves inhalation of a radioactive gas (i.e., Xenon-133) or radioaerosol (i.e., Tc-99m DTPA) for ventilation evaluation, and intravenous administration of radioactive particles which are trapped by the capillary bed (i.e., Tc-99m macroaggregated albumin) for perfusion. A PE should lead to a segmental perfusion defect which extends to the pleural surface with normal ventilation, labeled a mismatched defect. Using classic probabilistic reporting criteria, such as Prospective Investigation of Pulmonary Embolism Diagnosis (PIOPED) and PIOPED II, a scan is high probability for PE if there are at least two large mismatched perfusion defects, or the equivalent of allowing for summation of two moderate defects to equal one large defect. Overall probability depends on pretest probability as well; slightly more than half of high-probability VQ scans are due to PE in the setting of low clinical probability. Other criteria such as PISAPED and perfusion-only modified PIOPED II use a radiograph for ventilation evaluation, and do not use probabilistic reporting, replacing “high probability for PE” with “PE present.” The addition of single-photon emission computed tomography (SPECT) imaging may improve diagnostic accuracy.
Ventilation abnormality. Pulmonary ventilation and perfusion are coupled with gravity resulting in more perfusion and ventilation in the bases. Decreased aeration leads to reflex vasoconstriction allowing for blood to be shunted to aerated portions of the lungs. Therefore, processes primarily causing ventilation abnormalities, such as pneumonia, atelectasis, pulmonary edema, and chronic obstructive pulmonary disease (COPD), often result in matched defects. Probability of PE in this case is based on the number and size of matched defects ranging from very low to intermediate probability, using modified PIOPED criteria. Decreased perfusion can result in reflex bronchoconstriction, but it is transient. Comparison to a chest radiograph is important as a triple match, a VQ defect with a similar in size or smaller matched radiographic abnormality, is intermediate probability for PE if in the lower lungs. Emphysema can present with perfused lung between the defect and the pleura, known as the “stripe sign.”
Mass effect. Mass effect from effusion, tumor, or adenopathy can result in a VQ defect which is usually not, but may be, segmental. Effusion can cause compressive atelectasis resulting in reflex vasoconstriction and a matched defect. When an effusion layers within a fissure, it can result in a linear perfusion defect running along the fissure, known as the “fissure sign.” The concept of a triple match remains important with effusion as around one-third of patients with PE have anipsilateral effusion.
Diagnosis
High probability for PE.
✓ Pearls
At least two large segmental mismatched defects on a VQ scan is a sign of high probability for PE.
Triple matched defect is intermediate probability for PE in the lower lungs, and low probability in the upper region.
Perfused lung between a defect and the pleura (stripe sign) is seen with emphysema and not PE.
Suggested Readings
Parker JA, Coleman RE, Grady E, et al; Society of Nuclear Medicine. SNM practice guideline for lung scintigraphy 4.0. J Nucl Med Technol. 2012; 40(1):57–65 Roach PJ, Bailey DL, Harris BE. Enhancing lung scintigraphy with single-photon emission computed tomography. Semin Nucl Med. 2008; 38(6):441–449 Tunariu N, Gibbs SJ, Win Z, et al. Ventilation-perfusion scintigraphy is more sensitive than multidetector CTPA in detecting chronic thromboembolic pulmonary disease as a treatable cause of pulmonary hypertension. J Nucl Med. 2007; 48(5):680–684Case 37

Key Finding
Unilateral absent lung perfusion
Top 3 Differential Diagnoses for Perfusion Worse Than Ventilation
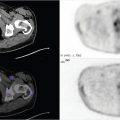
External compression (tumor, fibrosing mediastinitis). Unilateral absence of lung perfusion is a rare finding. A ventilation (V)/perfusion (Q) scan cannot determine the cause on its own. However, extrinsic compression of the pulmonary artery is the most common cause of a perfusion defect which is worse than ventilation. Typically, it is from a central lung tumor. Fibrosing mediastinitis is another entity that could lead to pulmonary artery occlusion and absence of perfusion. Cross-sectional imaging is essential to determine the presence of external compression.
Pulmonary artery anomalies or corrected congenital heart disease. Pulmonary artery atresia, severe pulmonary artery stenosis, or pulmonary webs may cause unilateral absence of perfusion with perfusion defect worse than ventilation. Additionally, corrected congenital heart disease may produce similar findings on V/Q scan. Dedicated contrast-enhanced cross-sectional imaging via CT or MRI/MRA can greatlyimprove detection, as well as correlation for prior surgical history.
Massive unilateral pulmonary embolism and aortic dissection. While massive unilateral pulmonary embolism (PE) can produce unilateral absent perfusion, it is less likely in comparison to other causes. Massive PE of this magnitude will often have more than one V/Q mismatch affecting both lungs. Absent perfusion of an entire lung is low probability of PE as per PIOPED II criteria. Hemorrhage from aortic dissection can result in pulmonary artery compression.
Top 3 Differential Diagnoses for Ventilation Worse Than Perfusion
Mucus plug. It may obstruct ventilation with compensatory vasoconstriction of the pulmonary arteries. The degree of accompanying perfusion abnormality will increase with time with continued obstruction due to arteriolar constriction induced by local hypoxia. As this is primarily an airway abnormality, the ventilation defect will be larger than the perfusion defect.
Unilateral diffuse parenchymal disease. Diffuse air space disease, atelectasis, or large pneumothorax involving an entire lung will result in decreased or absent ventilation with compensatory vasoconstriction of the pulmonary arteries. The degree of lung involvement will determine the degree of perfusion and ventilation defects.
Endobronchial lesion (mass or foreign body). They can cause a ventilation defect which is larger than perfusion. Central lesions can cause diminished activity within an entire lung. The relative decrease in ventilation to perfusion is an indicator of the extent of luminal involvement.
Diagnosis
Extrinsic compression (left hilar malignancy).
✓ Pearls
Ventilation scan may be performed with Xe-133 gas or Tc-99m diethylenetriamine pentaacetic acid (DTPA) aerosol.
Wedge-shaped (peripheral), segmental mismatched perfusion defects are concerning for PE.
Unilateral absent or decreased lung perfusion or ventilation is usually seen with nonembolic etiologies.
Suggested Readings
Pickhardt PJ, Fischer KC. Unilateral hypoperfusion or absent perfusion on pulmonary scintigraphy: differential diagnosis. AJR Am J Roentgenol. 1998; 171(1):145–150 Slonim SM, Molgaard CP, Khawaja IT, Seldin DW. Unilateral absence of right-lung perfusion with normal ventilation on radionuclide lung scan as a sign of aortic dissection. J Nucl Med. 1994; 35(6):1044–1047 White RI, Jr, James AE, Jr, Wagner HN, Jr. The significance of unilateral absence of pulmonary artery perfusion by lung scanning. Am J Roentgenol Radium Ther Nucl Med. 1971; 111(3):501–509Case 38

Clinical History
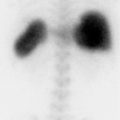
80-year-old female with pulmonary hypertension referred for pulmonary ventilation and perfusion scan to evaluate for evidence of chronic thromboembolic disease (▶Fig. 38.1).
Key Finding
Heterogeneous pulmonary perfusion
Top 3 Differential Diagnoses
Primary pulmonary hypertension (PPH). Elevation of the pulmonary arterial pressure due to elevated pressures in the pulmonary arterial system itself is known as primary (or precapillary) pulmonary hypertension. This condition has multiple etiologies, but can be idiopathic. The common feature for all causes is a progressive vasculopathy involving the pulmonary arterioles, responsible for the heterogeneous pattern of perfusion. There may also be a reversal of the normal gradient with the apices receiving more blood flow. It is progressive and can be fatal. A right heart catheterization with a mean pulmonary arterial pressure of 25mm Hg or greater is diagnostic. Ventilation and perfusion (VQ) scans are usually done to exclude chronic thromboembolic disease as a cause of pulmonary hypertension utilizing the same diagnostic criteria as for acute pulmonary embolism (PE). A normal VQ scan essentially excludes chronic thromboembolic disease as the etiology.
Congestive heart failure. Postcapillary pulmonary hypertension can be caused by anything that leads to elevated pulmonary venous pressures. In congestive heart failure, the increased pressure in the left ventricle leads to increased pressure in the left atrium and subsequently increased pulmonary venous pressure. This physiology is responsible for the characteristic radiographic findings including vascular enlargement and indistinctness. If left untreated, the process can lead to arteriolar damage from prolonged increased pressure, a combined pre- and postcapillary picture.
Vasculitis/fat emboli. Pulmonary vasculitis can be seen in the setting of many systemic and primary pulmonary conditions such as systemic lupus erythematosus, rheumatoid arthritis, and polyarteritis nodosa. Any of the vasculitides affecting the small- and medium-sized vessels will result in a heterogeneous pattern. Fat emboli are usually seen in the setting of long bone fracture.
Additional Considerations
COPD: As COPD progresses, the normal perfusion scan can become heterogeneous due to reflex vasoconstriction as well as parenchymal destruction.
Quantum mottle: Ensure correct radioisotope window, collimator, patient to camera distance, and imaging time. Can also occur if there is not enough radiopharmaceutical at the target site.
Diagnosis
COPD with pulmonary arterial hypertension.
✓ Pearls
A normal perfusion scan excludes chronic thromboembolism as the cause of pulmonary hypertension.
COPD will have normal perfusion early but heterogeneous perfusion as disease progresses.
Poor imaging statistics (quantum mottle) can simulate heterogeneous pulmonary perfusion.
Suggested Readings
Fukuchi K, Hayashida K, Nakanishi N, et al. Quantitative analysis of lung perfusion in patients with primary pulmonary hypertension. J Nucl Med. 2002; 43(6):757–761 Peña E, Dennie C, Veinot J, Muñiz SH. Pulmonary hypertension: how the radiologist can help. Radiographics. 2012; 32(1):9–32 Tunariu N, Gibbs SJR, Win Z, et al. Ventilation-perfusion scintigraphy is more sensitive than multidetector CTPA in detecting chronic thromboembolic pulmonary disease as a treatable cause of pulmonary hypertension. J Nucl Med. 2007; 48(5):680–684Case 39
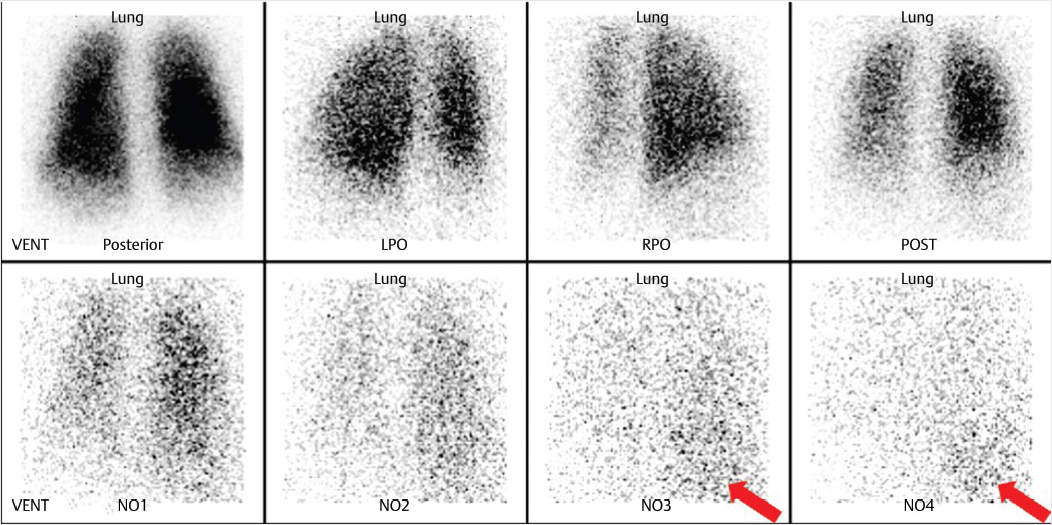
Clinical History
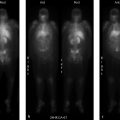
44-year-old female who presented with chest tightness, shortness of breath, and left lower extremity edema after recent long-distance travel. Left lower extremity ultrasound demonstrated a deep venous thrombosis in the distal superficial femoral vein (▶Fig. 39.1).
Key Finding
Liver uptake on Xenon-133 pulmonary ventilation scan
Diagnosis
Hepatic steatosis. Xenon-133 (Xe-133) is a radioactive noble gas which is produced by the fission of Uranium-235. It is one of the agents commonly used for pulmonary ventilation imaging. Administration of Xe-133 involves delivery of the gas through a closed system. The patient is first instructed to take a breath in and hold it for an initial breath-hold image, then free breaths Xe-133 for 3–5 minutes while additional images are obtained. The Xe-133 is then shut off and the patient continues to breathe for an additional 3–5 minutes allowing for washout imaging. Some of the radioactive gas will enter the alveolar walls and pulmonary capillaries leading to the pulmonary venous system. Once in the blood stream, organ-specific uptake of Xe-133 is determined by blood flow and tissue solubility. Xe-133 is highly soluble in fat. With diffuse fatty liver, slow progressive hepatic uptake will be seen toward the end of the ventilation study. Xe-133 has been used to both diagnose and assess fatty liver disease. It has proven utility for both alcoholic and nonalcoholic fatty liver disease (NAFLD) and has been shown to be more sensitive and specific than ultrasound. While liver biopsy remains the gold standard for diagnosis of NAFLD, an Xe-133 scan offers a reliable and noninvasive way to evaluate for this disease. This is increasingly relevant as NAFLD has become the most common cause of chronic liver dysfunction in Western countries. Qualitative assessment of liver uptake involves visually comparing the uptake in the liver seen on washout images to the peak activity seen in the lungs. Mechanisms for quantitative assessment have also been used, some of which require a technetium-99m sulfur colloid (Tc-99m SC) scan to be performed after the washout phase of the Xe-133 study to verify positioning of the liver for proper region of interest placement. Quantitative measures have been shown to accurately predict the level of fatty replacement. Despite the utility of Xe-133 in the evaluation of fatty liver disease, it is not performed frequently as the noninvasive evaluation has mostly been replaced by ultrasound, CT, and MRI.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree