Part 3 Cardiac
Case 22

Clinical History
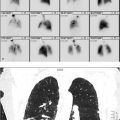
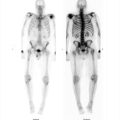
55-year-old male with known coronary artery disease post pacemaker placement (▶Fig. 22.1).
Key Finding
Myocardial perfusion defect which is worse on stress than rest images (reversible defect)
Top 2 Differential Diagnoses
Ischemia. Single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) remains the most common exam performed in the majority of Nuclear Medicine departments. MPI is generally performed for risk stratification for possible coronary artery disease, to help determine which patients need cardiac catheterization, and possible reperfusion. Most commonly used radiopharmaceuticals are the mitochondrial imaging agents, Tc-99m sestamibi, and Tc-99m tetrofosmin, having mostly replace thallium-201 (Tl-201) due to more advantageous imaging and dose characteristics. For the Tc-99m-based mitochondrial agents a 1-day protocol is frequently used for patient and scheduling convenience. SPECT imaging is obtained at rest first after administration of around 8–11 mCi of the radiotracer. Either pharmacologic or exercise stress is then performed, and a stress dose of around 25–30 mCi is injected at the appropriate time with subsequent repeat SPECT imaging. A perfusion defect present only on the poststress images, or a “reversible” defect, is concerning for stress-induced ischemia and suggests the presence of coronary artery disease (CAD) while providing prognostic value for risk of a major cardiac event. Other stress-induced findings associated with CAD include increased pulmonary or right ventricular (RV) uptake, increased poststress left ventricular volumes (transient ischemic dilation), and decreased poststress ejection fraction. MPI has good sensitivity for identifying CAD that results in greater than 50% luminal narrowing.
Shifting attenuation. Attenuation is always a concern when reviewing MPI. The photons emitted from the injected radiopharmaceutical can be attenuated by the patient, as well as by anything overlying the patient, resulting in an apparent perfusion defect. Common causes for attenuation defects include the breasts, chest wall, diaphragm, implanted pacemaker/defibrillator, electrocardiography (EKG) leads, and implanted medication ports. Attenuation most frequently results in a defect that is worse on the rest images than the stress images, when using the 1-day protocol, since approximately one-third of the amount of radioactivity is used for the rest exam. Breast attenuation usually results in an anteroseptal defect, and diaphragm in an inferolateral defect. A defect can confidently be attributed to attenuation when it is in a common location, is worse on rest than stress, does not have a corresponding wall motion abnormality, and attenuation is seen on the rotating raw images. Attenuation can result in an apparent reversible defect when the cause of attenuation shifts in location between the rest and stress images. If this is considered, repeat stress images obtained in the prone position, or SPECT/CT with attenuation correction, can be used for problem solving, as long as the patient is still available for imaging.
Diagnosis
Myocardial ischemia. Subsequent cardiac catheterization revealed diffuse plaque in the proximal to mid left anterior descending coronary artery resulting in over 50% stenosis.
✓ Pearls
A reversible myocardial perfusion defect, seen on stress but not rest images, is concerning for ischemia.
Shifting attenuation can result in an apparent reversible defect.
Prone imaging and attenuation correction with SPECT/CT are possible problem-solving techniques.
Attenuation will usually result in a defect that is worse on rest than stress and without abnormal motion, when using a 1-day protocol.
Suggested Readings
Hage FG, Ghimire G, Lester D, et al. The prognostic value of regadenoson myocardial perfusion imaging. J Nucl Cardiol. 2015; 22(6):1214–1221 Higgins JP, Higgins JA, Williams G. Stress-induced abnormalities in myocardial perfusion imaging that are not related to perfusion but are of diagnostic and prognostic importance. Eur J Nucl Med Mol Imaging. 2007; 34(4):584–595 Holder L, Lewis S, Abrames E, Wolin EA. Review of SPECT myocardial perfusion imaging. J Am Osteopath Coll Radiol. 2016; 5(3):5–13Case 23

Clinical History
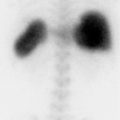
71-year-old male with ischemic cardiomyopathy and worsening exertional fatigue and dyspnea (▶Fig. 23.1).
Key Finding
Myocardial perfusion defect present on both rest and stress images (fixed defect)
Top 3 Differential Diagnoses
Infarct. A perfusion defect on single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) is generally described in terms of size, severity, location, and reversibility. A defect that is present on both the rest and stress images, or a “fixed” defect, is concerning for infarct. A true infarct should be associated with a wall motion abnormality on gated images. No differential is necessary if there is dyskinesia in the region of the fixed perfusion defect as that suggests the presence of a transmural infarct with aneurysm formation. There is some suggestion that the severity of a perfusion defect on SPECT MPI, particularly with Thallium-201 (Tl-201), can help predict the amount of infarcted tissue within the perfusion defect.
Hibernating myocardium. Coronary artery occlusion can lead to a spectrum of results depending on severity and duration of occlusion. Short-duration ischemia can lead to myocardial stunning which presents as an area with normal perfusion but abnormal motion. This will spontaneously resolve. Long-term repetitive episodes of ischemia can lead to functional downregulation to promote myocyte survival by decreasing oxygen demand, known as hibernating myocardium. This is often indistinguishable from infarct on SPECT MPI, with possible distinction by the defect severity. However, differentiation may be important as hibernating myocardium is viable and will likely respond to revascularization. If determining the extent of hibernating versus infarcted myocardium is clinically warranted, most frequently needed in the setting of a reduced ejection fraction (EF) with a fixed defect, further evaluation can be done with Tl-201, fluorine-18 fluorodeoxyglucose (F-18 FDG), and cardiac MRI. Hibernating myocardium will show redistribution on 24-hour Tl-201 images, and uptake (occasionally increased relative to the remaining myocardium) on F-18 FDG images. Subendocardial delayed enhancement on cardiac MRI that involves less than 50% of the wall thickness is considered a marker for viability.
Attenuation. Soft-tissue attenuation that remains in a fixed position can result in a fixed perfusion defect. This is commonly seen secondary to breast attenuation in the anterior/anteroseptal wall, and due to diaphragm attenuation in the inferior/inferolateral wall. Characteristics that suggest a perfusion defect is secondary to attenuation include location (often a nonvascular distribution), frequently worse on rest than stress with a 1-day protocol due to the lower administered dosage at rest, and wall motion and thickness are preserved.
Diagnosis
Apical infarct with aneurysm.
✓ Pearls
Hibernating myocardium is dysfunctional with reduced perfusion but will respond to revascularization.
Differentiating between viable and nonviable myocardium may be needed in the setting of reduced EF.
F-18 FDG, Tl-201, and MR imaging can all be used to asses for myocardial viability.
A fixed defect that has normal wall motion and thickening is likely secondary to attenuation.
Suggested Readings
Dvorak RA, Brown RK, Corbett JR. Interpretation of SPECT/CT myocardial perfusion images: common artifacts and quality control techniques. Radiographics. 2011; 31(7):2041–2057 Mc Ardle BA, Beanlands RS. Myocardial viability: whom, what, why, which, and how? Can J Cardiol. 2013; 29(3):399–402Case 24

Clinical History
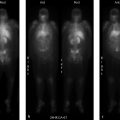
73-year-old female referred for myocardial perfusion imaging (MPI) because of nonsustained supraventricular tachycardia on mobile telemetry (▶Fig. 24.1).
Key Finding
Resting myocardial perfusion defect that improves after stress (“reverse redistribution/perfusion”)
Top 3 Differential Diagnoses
Attenuation/shifting attenuation. Myocardial perfusion is most frequently performed for risk stratification of patients with suspected or known coronary artery disease (CAD). Stress images are the most important in this setting, as recognition of a stress-induced perfusion defect raises the concern for ischemia, possibly requiring coronary angiography and revascularization. While most facilities use a 1-day protocol, performing reduced dose rest imaging pri- or to either exercise or pharmacologic stress the same day, the 2-day protocol includes stress imaging first. If the stress images are normal, rest of the images are likely not necessary as they are primarily used to provide specificity to a stress defect. However, occasionally a perfusion defect will be seen at rest that improves after stress, termed “reverse redistribution” or “reverse perfusion.” A possible cause is shifting attenuation. If an attenuating structure changes position between the rest and stress images, then the resulting defects will not be matched. Evaluation of the rotating raw images can help to visualize shifting attenuation. Attenuation on its own, that does not shift, can cause a defect that appears worse on rest than stress with 1-day protocol imaging due to the reduced dosage used for rest images.
Prior revascularization. A rest perfusion defect that improves with stress can be seen in patients post revascularization. The etiology for this phenomenon is not completely clear. It may be secondary to slow flow through the revascularized vessel, stent, or graft. Theoretically, stress-induced vasodilation distal to the revascularization results in a greater pressure differential leading to sufficient flow through a conduit that is otherwise insufficient. Reverse perfusion may also be due to more rapid washout of radiotracer from hibernating or stunned myocardium. This finding was originally thought to represent effective reperfusion; however, some studies have shown patients with postreperfusion reverse redistribution have a higher risk for cardiac events even in the setting of proven myocardial viability.
Cardiac sarcoid. Sarcoidosis is a multisystem disease of unknown etiology resulting in formation of noncaseating granulomas. Around 1 in 20 patients with sarcoidosis will have cardiac involvement, which may be asymptomatic or lead to sudden death. Reverse redistribution with cardiac sarcoid is thought to be secondary to constriction of coronary arterioles around granulomas. Fluorine-18 fluorodeoxyglucose (F-18 FDG) and cardiac MRI (cMRI) are better imaging agents for cardiac sarcoid and more frequently used.
Diagnosis
Attenuation (the defect resolved with attenuation correction, not shown).
✓ Pearls
If a reverse perfusion pattern is seen, raw data should be evaluated for attenuation/shifting attenuation.
Reverse perfusion post reperfusion may be due to slow flow requiring distal dilation to normalize.
Reverse perfusion can be seen with cardiac sarcoid, better evaluated with F-18 FDG positron emission tomography (PET) and cMRI.
Suggested Readings
Schatka I, Bengel FM. Advanced imaging of cardiac sarcoidosis. J Nucl Med. 2014; 55(1):99–106 Schillaci O, Tavolozza M, Di Biagio D, et al. Reverse perfusion pattern in myocardial spect with 99mTc-sestaMIBI. J Med Life. 2013; 6(3):349–354 Swinkels BM, Hooghoudt TE, Schoenmakers EA, Zinder CG, de Boo TM, Verheugt FW. Clinical significance of reverse redistribution on technetium-99m tetrofosmin single-photon emission computed tomography: an 18-month follow-up study. Neth Heart J. 2003; 11(3):113–117 Tanaka R, Nakamura T, Chiba S, et al. Clinical implication of reverse redistribution on 99mTc-sestamibi images for evaluating ischemic heart disease. Ann Nucl Med. 2006; 20(5):349–356Case 25

Clinical History
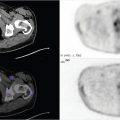
82-year-old female with new onset left bundle branch block (LBBB) and shortness of breath referred to evaluate for ischemia (▶Fig. 25.1).
Key Finding
Paradoxical bouncing motion of the interventricular septum during diastole, or “septal bounce”
Top 3 Differential Diagnoses
Ventricular interdependence. Septal bounce is a wall motion abnormality that may be seen on gated single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) along with other imaging modalities that allow evaluation of cardiac wall motion to include echocardiography, cardiac CT and cardiac MRI (cMRI). During early diastole, the septum will show a paradoxical bouncing motion, initially moving toward and then away from the left ventricle (LV). This motion abnormality suggests restricted ventricular filling which results in a relatively fixed volume for the heart. This equates to ventricular interdependence, i.e., changes in volume and pressure of one ventricle are propagated through the septum into the other ventricle. This is most commonly seen with constrictive pericarditis, but can also be associated with cardiac tamponade.
Left bundle branch block. Delayed conduction from a LBBB results in delayed LV activation. This alone can be associated with decreased LV function, even with normal myocyte contractility. Several septal wall motion abnormalities have been associated with LBBB, one of which is the septal bounce. Correlation with EKG obtained during performance of MPI can confirm the diagnosis.
Pulmonary hypertension. Chronic pulmonary hypertension can result in paradoxical motion of the interventricular septum due to elevated right ventricle (RV) pressure. Pressure in the LV drops to near zero in early diastole allowing the greater RV pressure to push the septum toward the LV center.
Diagnosis
Left bundle branch block.
✓ Pearls
Paradoxical bouncing of the septum toward the LV during diastole is known as a septal bounce.
Ventricular interdependence due to constrictive pericarditis or tamponade can cause a septal bounce.
Septal bounce can be due to delayed electrical activation of the LV in LBBB.
Exercise or dobutamine stress should be avoided with LBBB due to a possible false septal defect.
Suggested Readings
Breithardt G, Breithardt OA. Left bundle branch block, an old-new entity. J Cardiovasc Transl Res. 2012; 5(2):107–116 Dvorak RA, Brown RK, Corbett JR. Interpretation of SPECT/CT myocardial perfusion images: common artifacts and quality control techniques. Radiographics. 2011; 31(7):2041–2057 Walker CM, Chung JH, Reddy GP. “Septal bounce.” J Thorac Imaging. 2012; 27(1):W1Case 26

Clinical History
80-year-old female with history of chronic obstructive pulmonary disease and atrial fibrillation presenting with chest pain (▶Fig. 26.1).
Key Finding
Transient ischemic dilation
Top 3 Differential Diagnoses
Multivessel coronary artery disease. Myocardial perfusion imaging (MPI) is designed to detect areas of ischemia which may benefit from reperfusion. Ischemia is diagnosed by noting a reversible defect, decreased activity in a portion of the myocardium on post-stress imaging (exercise or chemical stress) in comparison to rest imaging. With multivessel balanced ischemia, however, focal regions of decreased activity may not be apparent as the images are normalized to the “hottest” pixels. In these situations, diffuse subendocardial ischemia may cause apparent dilation of the left ventricle (LV) cavity on post-stress imaging. This is an important finding to make as it may be the only sign of ischemia and can be associated with high-grade stenosis.
Hypertensive heart disease. Global subendocardial ischemia, resulting in apparent dilation of the LV cavity on poststress imaging, may also be caused by severe hypertensive heart disease. This is likely a multifactorial process, but elevated end diastolic pressures in the LV are contributory as this leads to an increase in the required filling pressure of the epicardial coronary vessels. This can cause TID without any associated coronary artery stenosis.
Dilated cardiomyopathy. TID can again be seen in the absence of coronary artery disease (CAD) rarely in patients with dilated cardiomyopathy. The subendocardial hypoperfusion in this case is likely due to decreased coronary flow reserve.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree