The glossopharyngeal, vagus, spinal accessory, and hypoglossal cranial nerves can be affected by an acute or chronic process that has an impact on the way the patient presents clinically. Knowledge of nerve pathways and relations to surrounding structures is fundamental when evaluating patients who have lower cranial nerve symptoms. A systematic “segment-based” approach helps to narrow the differential diagnosis Pathologic conditions that cause lower cranial nerve symptoms are presented.
Radiologic evaluation of the patient who has isolated or combined deficits of the lower four cranial nerves requires knowledge of the course of each nerve and the muscles innervated by these nerves. Because glossopharyngeal (CN IX), vagus (CN X), and spinal accessory (CN XI) nerves share some functions and nuclei, they are most often discussed as a group. There are some unique characteristics of each nerve that are also described, however. Although the hypoglossal nerve has a different skull base pathway, it is located in close proximity to the other lower cranial nerves. The severity of symptoms that result from insults to these nerves depends on the chronicity of the insult. If the cause is a slow-growing tumor or a chronic insult, the patient may adapt well, with only a partial palsy. If the event is acute, however, such as traumatic or postsurgical, the patient usually has severe complete paralysis.
In this article, a brief overview of the anatomy of the lower four cranial nerves is presented, because this topic has been discussed in detail elsewhere in this issue. The clinical manifestations associated with impairment of the lower four cranial nerves are also discussed, and the commonly associated pathologic conditions and their classic imaging findings are reviewed.
Anatomy and clinical manifestations
Glossopharyngeal Nerve—Cranial Nerve IX
CN IX is composed of motor and sensory fibers. By itself, the glossopharyngeal nerve provides motor innervation to a single muscle, the stylopharyngeus muscle. The sensory segment of CN IX forms the first synapse in the superior ganglion and enters the medulla to synapse further in the nucleus spinalis of the trigeminal nerve—CN V. Parasympathetic fibers of CN IX, originating in the inferior salivary nucleus, go to the parotid glands, stimulating gland secretion. There are also special sensory fibers, part of the nucleus solitarius, for taste from the posterior one third of the tongue.
After exiting the brain stem in the retro-olivary sulcus, CN IX crosses the cistern and exits the skull base through the pars nervosa of the jugular foramen. In the suprahyoid neck, CN IX runs in the high carotid space, medial to the styloid process and anterior to the internal carotid artery (ICA) and internal jugular vein.
As previous noted, given the close proximity of the glossopharyngeal nerve to the vagus and spinal accessory nerves, pathologic conditions commonly affect all three nerves simultaneously, often related to the so-called “jugular foramen (Vernet’s) syndrome”. Pathologic conditions affecting CN IX in isolation are rare. Patients usually present with external otalgia, slight dysphagia, and changes in taste from the posterior one third of the tongue. Physical examination demonstrates loss of the gag reflex on the affected side, deviation of the uvula to the opposite side, and differences in sensation between sides of the soft palate and pharynx. Because there are also parasympathetic fibers to the parotid gland and carotid body, secretions of saliva from the parotid gland are decreased and the heart rate may be accelerated ( Box 1 ). This last finding has only been described in patients immediately after surgical sectioning of the nerve.
Glossopharyngeal nerve (CN IX)
- •
External otalgia
- •
Slight dysphagia
- •
Loss of taste from the posterior one third of the tongue
- •
Loss of gag reflex on the affected side
- •
Decreased saliva secretion from the parotid gland
- •
Accelerated heart rate (only after surgical sectioning)
Vagus nerve (CN X)
- •
Soft palate droops on the affected side
- •
Deviation of the posterior pharyngeal wall on the affected side
- •
Hoarseness attributable to vocal cord paralysis
- •
Bilateral palsy results in a dilated and atonic esophagus, stomach, and intestine
Accessory nerve (CN XI)
- •
Shoulder droop secondary to trapezius muscle denervation
- •
Diminished strength on turning the head toward the paralyzed side
- •
Weakness elevating the shoulder above the horizontal plane
Hypoglossal nerve (CN XII)
- •
Compromised motor function of the tongue
Vagus Nerve—Cranial Nerve X
The vagus nerve is a long nerve extending from the brain stem to the splenic flexure of the colon, with branchial motor, visceral motor, general sensory, and visceral sensory functions. The branchial motor segment carries fibers to the laryngeal musculature, extralaryngeal neck muscles, and pharyngeal muscles. The vagus nerve originates from the nucleus ambiguus that continues cranially with the motor nuclei of the facial and trigeminal nerves. General sensory fibers come from the dura mater, external ear canal, tympanic membrane, and pharyngeal and laryngeal mucosa. Visceral sensory fibers originate from the intrathoracic and intra-abdominal structures, related to cardiovascular, respiratory, and gastrointestinal reflexes. The visceral motor (parasympathetic) fibers of the nerve go to glands of the pharynx and larynx, thoracic and abdominal viscera, and cardiac muscle.
The vagus nerve has three major branches: pharyngeal, superior laryngeal, and recurrent laryngeal. The pharyngeal branch is the principal motor nerve to the pharynx, supplying all muscles of the pharynx and soft palate, with the exception of the stylopharyngeus and tensor veli palatini muscles, which are supplied by CN IX and CN V3 (mandibular nerve), respectively. The superior laryngeal nerve divides into internal (mainly a sensory nerve from the hypopharynx and larynx above the vocal cords) and external (a motor nerve to the inferior constrictor and cricopharyngeus muscles) branches. The distal branch is the recurrent laryngeal nerve (RLN), which has different right and left pathways that are described subsequently.
The vagus nerve exits the brain stem in the retro-olivary sulcus of the medulla. Eight to 10 rootlets emerge, caudal to those of CN IX. After crossing the perimedullary cistern, the nerve exits the skull base through the pars vascularis of the jugular foramen, sharing the same dural sheath as the accessory nerve. In the high carotid space, the vagus nerve lies posterior to, and in a groove between, the internal jugular vein and the ICA. Descending within the carotid sheath, the vagus nerve gives branches to the pharynx, larynx, and constrictor muscles, as described previously. In the mediastinum, each vagus nerve takes a different pathway to reach the esophageal, cardiac, and pulmonary plexuses.
The right and left RLNs exit the vagus at different levels, which is extremely important to remember when evaluating patients who have vocal cord paralysis. The right RLN nerve exits higher, looping under the right subclavian artery before ascending in the right tracheoesophageal groove. The left RLN branches from the vagus in the thorax and loops under the aortic arch to ascend in the left tracheoesophageal groove. Because of these differing origins, it is essential to continue scanning caudally to the level of the aortopulmonary window when evaluating patients who have left vocal cord paralysis or the entire course of the left vagus nerve or RLN is not included on cross-sectional imaging examinations.
Patients who have unilateral vagal paralysis present with multiple findings. The soft palate droops and does not rise on phonation, and the palatal reflex is absent. Examination of the oropharynx demonstrates deviation of the posterior pharyngeal wall, known as the “side scenes phenomenon”. Hoarseness is present because of ipsilateral vocal cord paralysis. Pooling of secretions on the side of paralysis is usually identified during laryngoscopy. Dysphasia may be present because of swallowed food being pushed toward the paralyzed side of the hypopharynx by muscles of the intact side. Blood pressure and heart rate increase for approximately 2 months because of loss of parasympathetic innervation. Bilateral vagus nerve paralysis results in bilateral vocal cord paralysis and more severe dysphasia. Bilateral loss of visceromotor innervation results in dilatation and atony of the esophagus, stomach, and intestine (see Box 1 ).
When clinical findings implicate involvement of CN IX and CN X, the offending pathologic findings can be localized to the brain stem, subarachnoid cisterns, skull base, or suprahyoid carotid space. Isolated distal vagal neuropathy, manifested by isolated vocal cord dysfunction, places the pathologic findings below the level of the hyoid.
Spinal Accessory Nerve—Cranial Nerve XI
The spinal accessory nerve is purely motor, providing innervation to the sternocleidomastoid and trapezius muscles. The nerve has two separates components: cranial and spinal. The cranial component originates in the nucleus ambiguus, along with CN IX and CN X. The spinal component arises as multiple roots in the anterior horn of the spinal cord from C1 to C6. These fibers ascend within the spinal canal, passing posterior to the vertebral artery (VA), to enter the posterior cranial fossa. The two components course through the perimedullary cistern and exit the skull base through the pars vascularis of the jugular foramen along with nerve X. Within the high carotid space, the spinal accessory nerve courses lateral to the vagus nerve.
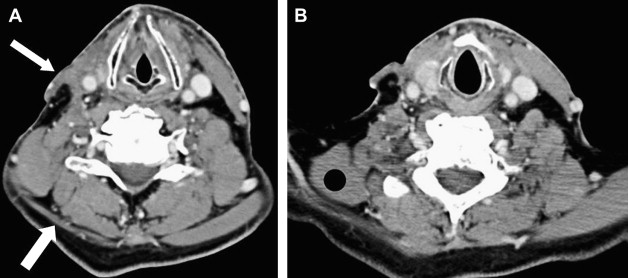
Because of the proximity of CN IX, CN X, and CN XI at the skull base, at the subarachnoid cisterns, and within the high carotid space, it is not surprising that patients who have CN XI palsy typically have involvement of other lower cranial nerves. Paralysis of the spinal accessory nerve is diagnosed clinically by ipsilateral shoulder droop, as a result of trapezius muscle denervation, and decreased strength on turning the head toward the paralyzed side, as a result of sternocleidomastoid denervation. There may also be weakness of shoulder elevation because of insufficient fixation of the scapula to the thorax wall (see Box 1 ).
An important finding of which the radiologist should be aware when interpreting studies of patients who have trapezius and sternocleidomastoid muscle atrophy or resection (ie, after radical neck dissection) is that there is often compensatory hypertrophy of the ipsilateral levator scapula muscle. This occurs as the muscle attempts to assume some of the functions of the denervated muscles and should not be confused with a mass ( Fig. 1 ).
Hypoglossal Nerve—Cranial Nerve XII
The hypoglossal nerve supplies motor innervation to the tongue and also contributes to innervation of the infrahyoid strap muscles through the ansa hypoglossi. The nucleus is located in the paramedian floor of the fourth ventricle, bulging into the ventricle, producing the hypoglossal trigone. The nerve courses anteriorly though the medulla, between the olive (laterally) and the medial longitudinal fasciculus, medial lemniscus, accessory olivary nucleus, and pyramidal tract (medially). Rootlets exit the brain stem in the preolivary sulcus and course posterolateral to the VA within the perimedullary cistern to exit the skull base through the hypoglossal (anterior condylar) canal. In the high nasopharyngeal carotid space, the nerve lies in the carotid sheath in close proximity to the other lower four cranial nerves. At the level of the mandible, the hypoglossal nerve loops anteriorly around the occipital artery, lying inferior to the posterior belly of the digastric muscle. It then runs along the surface of the hyoglossus muscle in the sublingual space, above the mylohyoid sling, to reach the tongue musculature.
Patients who have hypoglossal paresis present clinically with ipsilateral tongue paralysis and fasciculation (see Box 1). Approximately 4 to 6 weeks after injury, the intrinsic and extrinsic muscles of the tongue undergo atrophy and fatty replacement (see the article by Smoker and Reede elsewhere in this issue).
Pathologic findings of the lower cranial nerves
When paralysis of one or more of the lower four cranial nerves is identified, complete evaluation of the nerves from their brain stem nuclei to their “end organs” must be performed. For purposes of organization and discussion, this can best be accomplished by considering the nerves in five segments: (1) brain stem or nuclear segment; (2) cisternal segment, affecting the nerves as they transverse the cisterns; (3) skull base segment: jugular foramen and hypoglossal canal; (4) suprahyoid neck segment, compromising the nerves as they course through the nasopharyngeal and oropharyngeal carotid spaces; and (5) infrahyoid neck or mediastinal segment, only affecting the vagus nerve. With this organization, a reasonable differential diagnosis can be constructed for pathologic findings in each segment (ie, inflammatory or infectious, traumatic, miscellaneous) ( Box 2 ). It should be noted, however, that many lesions may affect the nerves in more than one segment (ie, nerve sheath tumors may involve any segment other than the nuclear segment, paragangliomas can be located in the suprahyoid and cisternal segments in addition to the skull base, aneurysms can also be located in more than one segment). In the following discussion, lesions are presented as they occur in their major segments.
Brain stem or nuclear segment
Vascular
Wallenberg’s syndrome (stroke)
Hemorrhage
Vascular malformations
Inflammatory
Multiple sclerosis (MS)
Lyme disease
Neoplastic
Gliomas
Lymphoma
Metastases
Ependymoma
Cisternal segment
Inflammatory
Tuberculosis
Sarcoidosis
Vascular
Vascular malformations
Vertebrobasilar dolichoectasia (VBD)
Posterior inferior cerebellar and VA aneurysm
VA dissection
Neoplastic
Leptomeningeal metastases
Leptomeningeal lymphoma
Nerve sheath tumors
Meningioma
Congenital
Chiari I
Skull base segment
Inflammatory
Osteomyelitis
Trauma
Skull base fractures
Neoplastic
Paragangliomas
Meningiomas
Nerve sheath tumors
Chordomas
Metastases
Nasopharyngeal carcinoma
Lymphoma
Suprahyoid segment
Inflammatory
Carotid space abscess
Neoplastic
Paragangliomas
Meningiomas
Nerve sheath tumors
Nasopharyngeal carcinoma
Vascular
ICA dissection and aneurysm
Miscellaneous
Second branchial cleft cyst
Infrahyoid or mediastinum segment
Inflammatory
Mediastinitis
Inflammatory adenopathy
Neoplastic
Bronchogenic carcinoma
Metastases
Lymphoma
Vascular
Aortic aneurysm
Cardiomegaly
Miscellaneous
Bronchogenic cyst
Brain Stem or Nuclear Segment
Vascular, neoplastic, and inflammatory lesions are the most common pathologic findings to involve the brain stem segment. The brain stem receives its arterial supply from the posterior circulation. The medulla is supplied anteromedially and anterolaterally by the VA and anterior spinal arteries. Laterally and posteriorly, the medulla is supplied by the posterior inferior cerebellar artery (PICA). Occlusion of the VA or PICA can cause a lateral medullary infarct, clinically described as the Wallenberg syndrome ( Fig. 2 ). This syndrome is composed of the following clinical signs: difficulty in swallowing, hoarseness, dizziness, nausea, vomiting, nystagmus, and balance or gait incoordination. Because the infarct also affects the descending sympathetic pathway, Horner’s syndrome is usually present. Involvement of the nucleus of CN V and lateral spinothalamic tract produces loss of pain and temperature to the ipsilateral face and contralateral limbs and trunk.
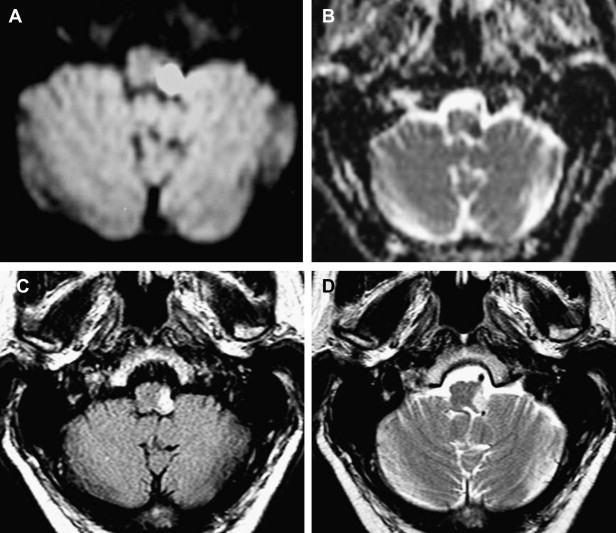
Diffusion-weighted imaging (DWI) is recommended for evaluation of brain stem infarcts. False-negative diffusion of lateral medullary strokes has been described within the first 24 hours, however. This is probably attributable to the low resolution of DWI echo planar imaging for detection of small lesions. If symptoms persist, the study should be repeated.
Primary hemorrhage of the medulla is rare. Brain stem hemorrhage is often restricted to the pons. Hypertension and vascular malformations are the most common causes. Anticoagulation has also been described as a cause of primary brain stem hemorrhage. Vascular malformations, such as cavernomas and arteriovenous malformations, can cause lower cranial nerve palsies, commonly associated with other neurologic symptoms, especially when they bleed and produce mass effect.
Metastases, gliomas, and lymphoma are the principal neoplasms to affect the brain stem and lower cranial nerve nuclei. The nerve palsy can be bilateral if the tumor is midline, affecting both nuclei, or unilateral when the lesion is eccentric or exophytic. Syringobulbia associated with ependymoma has also been reported to cause lower cranial nerve paralysis.
MS can involve the brain stem and is reported to affect 30% of patients at the onset of disease. The pons is the most commonly affected site, followed by the midbrain and, less commonly, the medulla. In patients who have lower cranial nerve involvement, the vagus nerve is the most commonly affected cranial nerve at the onset of disease. An infectious etiology that may mimic MS is Lyme disease, and it can rarely cause lower cranial nerve palsy.
Cisternal Segment
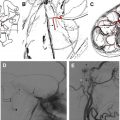
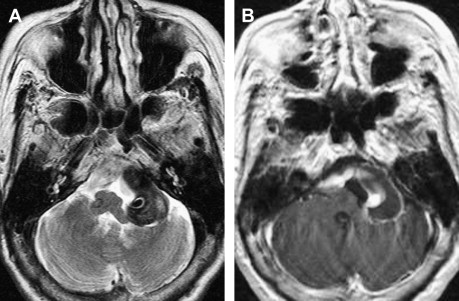
Major pathologic findings that affect the lower cranial nerves in their cisternal segments include vascular, infectious, neoplastic, and congenital lesions. VBD refers to elongation and dilatation of the vertebrobasilar vessels typically associated with hypertension. Although the pathogenesis of VBD is unclear, a vessel wall abnormality has been implicated. VBD can be asymptomatic or can produce cranial nerve symptoms attributable to compression. Facial and trigeminal nerves are most commonly affected, resulting in hemifacial spasm or trigeminal neuralgia, but isolated lower cranial nerve palsies have also been reported. Aneurysms of the VA and PICA, especially fusiform aneurysms, and vascular malformations within the cisterns can also cause lower cranial nerve symptoms produced by the mass effect ( Fig. 3 ). Isolated hypoglossal palsy has been reported in association with VA dissection.
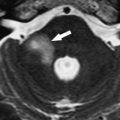
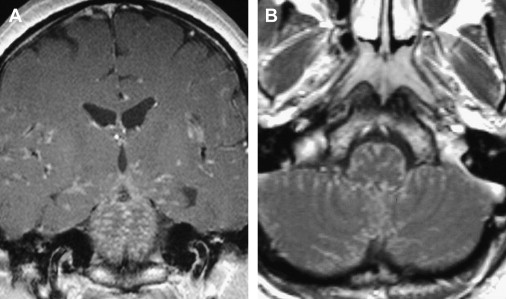
Various infectious and inflammatory processes that have a predisposition to affect the cisterns around the brain stem may also cause lower cranial nerve impairment, particularly granulomatous diseases. Tuberculous meningitis preferentially involves the basal cisterns and sylvian fissures and coats the cerebellum and brain stem with a thick gelatinous exudate. Cranial nerve palsies occur in 20% to 30% of patients and may be the presenting manifestation. Sarcoidosis frequently affects the central nervous system, with symptoms from leptomeningeal involvement being the most common manifestation, present in 40% of patients. Leptomeningeal sarcoidosis has a similar appearance to leptomeningeal tuberculosis. There is poor correlation between the imaging findings and the clinical symptoms in sarcoidosis, because some patients have symptoms without imaging findings and some asymptomatic patients have prominent imaging findings of cisternal involvement. As described with tuberculosis, any cranial nerve can be involved. MR imaging is more sensitive than CT for demonstration of the abnormal findings, with thickening and enhancement of the leptomeninges seen on postcontrast T1-weighted images ( Fig. 4 ). Idiopathic hypertrophic pachymeningitis is a rare entity, essentially a diagnosis of exclusion, that can also cause lower cranial nerve palsies. In summary, any inflammation or infection affecting the subarachnoid cisterns around the medulla may produce lower cranial nerve symptoms.
Central nervous system lymphoma can affect the cranial nerves when leptomeningeal spread of disease is present. The radiologic appearance is similar to that of leptomeningeal sarcoidosis or tuberculosis. Meningiomas ( Fig. 5 ) and nerve sheath tumors ( Fig. 6 ) can originate or extend to involve the cisternal segment. These are further discussed in the segment on the skull base in this article.
Lower cranial nerve dysfunction, such as vocal cord paralysis and tongue hemiatrophy, has been reported to be present in 20% of patients who have the Chiari I malformation. Controversy exists regarding the exact mechanism related to this phenomenon. One proposed mechanism is that traction and compression of the lower cranial nerves is attributable to herniation of the cerebellar tonsils through the foramen magnum ( Fig. 7 ).
Skull Base Segment
Neoplasms (benign and malignant), infections, or trauma injuries may damage the lower cranial nerves as they traverse the skull base. Three main neoplasms are located in the vicinity of the jugular foramen: paragangliomas, nerve sheath tumors, and meningiomas.
Paragangliomas are highly vascular lesions arising from nonchromaffin cells of neural crest origin. There are four common locations for these neoplasms within the head and neck: carotid artery bifurcation (carotid body paraganglioma), along the vagus nerve (vagal paraganglioma), at the jugular foramen (jugular paraganglioma), and in the middle ear (tympanicum paraganglioma). These neoplasms may occur sporadically or in an inherited familial pattern. Paragangliomas are hypervascular and show dense tumor staining on conventional angiography.
Jugular paragangliomas commonly affect the lower cranial nerves at the level of the skull base. They arise within the jugular foramen, from paraganglia surrounding the jugular bulb, Arnold’s nerve (branch of CN X), or Jacobsen’s nerve (branch of CN IX). MR imaging demonstrates a soft tissue mass; if the lesion is large enough, a salt and pepper appearance may be identified on standard spin echo imaging. The “salt” represents slow flow versus hemorrhage, and “pepper” represents flow voids within the mass. Jugular paragangliomas may extend to involve the middle ear cavity (jugulotympanicum paraganglioma). CT may be helpful in demonstrating a “moth-eaten” pattern of jugular foramen or spine erosion attributable to progressive growth of the tumor ( Fig. 8 ).
This destructive pattern is not associated with schwannomas, which remodel and expand the jugular foramen, or meningiomas, which are frequently associated with sclerosis.
Schwannomas and neurofibromas are benign primary neoplasms that can affect the cranial nerves. Approximately 40% of these tumors occur in the head and neck, and they can originate from any cranial nerve, including the lower four cranial nerves. Schwannomas and neurofibromas cannot be differentiated by their imaging characteristics; however, cranial nerve neurofibromas are extremely rare. Calcification and hemorrhage are uncommon, but cystic or fatty degeneration is frequently associated with schwannomas. As opposed to paragangliomas, which occur exclusively in the jugular canal, schwannomas can also arise within the hypoglossal canal. The clinical manifestations of these tumors, when located in the jugular foramen or hypoglossal canal, are variable, depending on tumor growth patterns. These neoplasms cause expansion and remodeling of the jugular foramen ( Fig. 9 ) or hypoglossal canal ( Fig. 10 ) without osseous destruction. As opposed to paragangliomas, extension to involve the middle ear is not a feature of these tumors. MR imaging signal characteristics of nerve sheath tumors are similar in all locations: isointense to hypointense signal on T1-weighted images; heterogeneous to homogeneous hyperintense signal on T2-weighted images; and variable enhancement, depending on the proportion of Antoni A and Antoni B cells and the amount of cystic degeneration.
Posterior fossa meningiomas account for 10% of intracranial meningiomas. They are described as occurring in six major locations: (1) cerebellar convexity, (2) cerebellopontine angle, (3) jugular foramen, (4) petroclival, (5) foramen magnum, and (6) unclassified. A review of 161 cases by Roberti and colleagues reported that 2 of 14 patients who had cerebellar convexity meningiomas, 1 of 9 patients who had cerebellopontine angle meningiomas, 2 of 7 patients who had jugular foramen meningiomas, 8 of 110 patients who had petroclival meningiomas, and 6 of 21 patients who had foramen magnum meningiomas had CN IX, CN X, and CN XI deficits. Additionally, 1 patient who had a cerebellar convexity meningioma, 1 patient who had a jugular foramen meningioma, 4 patients who had petroclival meningiomas, and 4 patients who had foramen magnum meningiomas had CN XII deficits. Meningiomas that originate in the posterior fossa can extend to involve the internal auditory canal, hypoglossal canal, or middle ear. They usually manifest isointense to hypointense signal on T1-weighted images, isointense signal on T2-weighted images, and enhancement on postcontrast T1-weighted images. Flow voids are not associated with these lesions. CT of meningiomas located around the jugular foramen often demonstrates sclerosis, which, together with the lack of vascular flow voids, is helpful in differentiating these tumors from paragangliomas ( Fig. 11 ).
Another tumor that can extend to involve the lower cranial nerves is the clivus chordoma. Although chordomas are histologically benign neoplasms, they are locally aggressive. They originate from embryonic notochordal remnants, most commonly encountered in the sacrum and clivus. Clivus chordomas can produce lower cranial nerve palsies as they extend to involve the skull base or, if they have an eccentric component, by abutting the nerves in their cisternal segments. CT typically shows an expansile, destructive, lytic lesion with an associated soft tissue mass centered within the clivus. Bone windows are helpful to define the osseous destruction. MR imaging is the best modality for the evaluation of chordomas because it provides excellent soft tissue contrast. T1-weighted MR imaging demonstrates a low signal intensity mass; however, some areas of high T1 signal may be identified as a result of intratumoral hemorrhage or mucous pools. The typical chordoma has characteristic high T2 signal intensity with low signal septations that represent the multilobulated feature of these lesions. Marked enhancement is present in most chordomas, described as a “honeycomb” appearance ( Fig. 12 ).
Metastatic disease can affect cranial nerves by perineural spread or skull base involvement. Skull base metastases from distant tumors occur in 4% of patients who have cancer. In a review of 43 skull base metastasis by Greenberg and colleagues, lung, prostate, and breast neoplasms were reported to be the most common primary neoplasms. Laigle-Donadey and colleagues reported cranial nerve palsies associated with skull base metastases as the presenting symptom in 28% of patients. MR imaging is the best modality to demonstrate marrow replacement and an enhancing skull base mass ( Fig. 13 ). CT is a complementary modality that better demonstrates the osseous destruction and is particularly helpful in differentiating fibrous dysplasia (FD) from metastases, a distinction that is often quite challenging on MR imaging.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree