Perineural spread (PNS) refers to the extent of tumor cells or other nonneoplastic lesions along the tissues of the nerve sheath, its overall incidence ranges from 2.5% to 5%. PNS is more frequently associated with carcinoma arising from minor or major salivary glands (more often adenoid cystic carcinoma), mucosal or cutaneous squamous cell carcinoma, basal cell carcinoma, melanoma, lymphoma, and sarcoma. Although PNS was previously associated with worsening prognosis, increasing evidence shows that cure is possible. Therefore, radiologists must be aware of the relevant cranial nerve anatomy and thoroughly scrutinize not only the nerves close to the primary tumor site but also the whole neural pathways that can be accessed by PNS. Equally critical is knowledge of the radiologic appearance of perineural tumor extension and the best imaging strategies to detect PNS.
Perineural spread (PNS) refers to the extent of tumor cells or other nonneoplastic lesions along the tissues of the nerve sheath. In head and neck malignancies, perineural spread has been described in surgical and imaging literature for decades.
Although PNS was previously associated with worsening prognosis, increasing evidence shows that cure is possible. In patients who have PNS from squamous cell carcinoma of the skin, a cure rate of approximately 80% can be achieved when both symptoms and imaging findings (for PNS) are absent.
In addition, in symptomatic patients the lesions limited to peripheral nerves or with minimal/moderate PNS tumor burden have a better local control rate, although a similar survival rate, compared with patients who have central or macroscopic disease.
This pattern of neoplastic spread can be clinically unsuspected while it conveys the tumor cells beyond the surgical and radiation fields of treatment. Therefore, if PNS is undetected or its extent is underestimated by imaging techniques, treatment will probably not control the disease.
Two additional points help radiologists understand the clinical significance of PNS assessment. First, in most head and neck neoplasms, the primary lesion and its gross extent have already been detected and estimated at clinical examination. Second, treatment of the neck is mainly dictated by the site and size of the primary tumor. Therefore, the evaluation of possible PNS through imaging may influence treatment planning more than identifying the primary tumor or assessing nodal disease.
Incidence, epidemiology, and most frequent malignant tumors showing perineural spread
Overall, the incidence of PNS ranges from 2.5% to 5%. Although it may occur with any head and neck malignancy, PNS is more frequently associated with carcinoma arising from minor or major salivary glands (more often adenoid cystic carcinoma [ACC]), mucosal or cutaneous squamous cell carcinoma (SCC), basal cell carcinoma (BCC), melanoma, lymphoma, and sarcoma.
PNS along a major (named) nerve has been observed in up to 28% of ACC arising from major or minor salivary and nonsalivary glands. Immunohistochemical studies have shown a high expression of neural cell adhesion molecules in ACC. These molecules could facilitate the mechanism of PNS.
Also the desmoplastic melanoma has a striking propensity to invade nerves. This ability has been related to a high expression of the p75 neurotrophin receptor (highly expressed also in a small series of ACC). During development, the interaction of nerve growth factor with the p75 receptor located on Schwann cells promotes the migration of Schwann cell to the nerve.
Although the adjacent nerves are invaded in up to 30% of desmoplastic melanoma, spread along cranial nerves is expected in no more than 1% to 2%.
Among tumors of the skin, SCC spreads more frequently along cranial nerves (5%–14%) than BCC, though in recurrent BCC the incidence of PNS significantly increases.
Even in the absence of neurologic abnormalities, accurate imaging is recommended to assess or rule out PNS along nerves whose distribution corresponds to the innervation of glands, mucosa, and skin areas of the face.
Therefore, radiologists must be aware of the relevant cranial nerve anatomy and examine not only the nerves close to the primary tumor site but also the whole neural pathways that can be accessed through PNS. Equally critical is the knowledge of the radiologic appearance of perineural tumor extension and of the best imaging strategies to detect PNS.
Neural pathways relevant for perineural spread
In most cases the peripheral branches of the second (V2) or third division (V3) of the trigeminal nerve and the descending branch of the facial nerve serve as conduits for perineural tumor spread into the skull base.
Preexisting neural pathways, such as the vidian nerve, greater superficial petrosal nerve (GSPN), and auriculotemporal nerve (ATN), account for neoplastic dissemination from trigeminal branches to those of the facial nerve (and vice versa). Less frequently, the ophthalmic nerve (V1) or hypoglossal nerve are involved. PNS along other nerve routes has been reported in few patients. Perineural tumor spread is usually antegrade (toward the central nervous system), but retrograde spread may also occur.
Trigeminal Nerve
The trigeminal nerve is the largest of the cranial nerves, with a diameter of approximately 2.5 mm when measured a few millimeters from the entry point into the pons. The nerve exits the ventral surface of the pons and traverses the prepontine cistern. Once it reaches the petrous apex, the nerve runs through a defect of the dura and enters Meckel’s cave, which is located lateral to the cavernous sinus. Meckel’s cave is a trigeminal cistern filled with cerebrospinal fluid (CSF) and enclosed by two dural layers: the external layer, derived from the internal layer of cranial periosteum, and the internal layer, the true meningeal dura mater.
The sensory rootlets of the trigeminal nerve reach the concave gasserian ganglion, placed on the floor of Meckel’s cave. The ganglion has a semilunar shape. The concave surface (posteromedial) is outlined by the CSF. From the (opposite) convex surface, which blends with the dura of the floor of Meckel’s cave, originate the three branches of the trigeminal nerve. The ophthalmic nerve (V1) and the maxillary nerve (V2) are purely sensory, whereas the mandibular nerve (V3) has both sensory and motor functions.
The ophthalmic nerve divides into three branches (lacrimal, frontal, and nasociliary nerves) immediately before entering the superior orbital fissure. These nerves supply branches to the lacrimal gland and conjunctiva; to the skin of the eyelids, eyebrow, forehead, and nose; to the cornea, ciliary body, and iris; and to the mucosa of the nasal cavity.
Although PNS along V1 can occur with malignancies arising from the mucosa investing the nasoethmoid complex or from intraorbital neoplasms, it is generally caused by cutaneous malignant neoplasms (SCC or BCC, melanoma). In this setting, the two terminal branches of the frontal nerve are the most likely to be invaded: the supraorbital nerve (largest branch, runs between the orbital vault and the levator palpebrae superioris, leaves the orbit through the supraorbital foramen) and the supratrochlear nerve (runs medially to the pulley for the superior oblique muscle).
Numbness or pain in the forehead or upper eyelid skin and conjunctiva are associated with involvement of the two terminal branches of the frontal nerve.
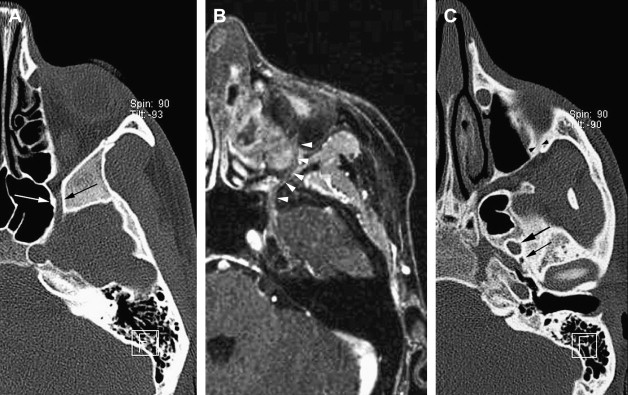
The maxillary nerve shows a particular bayonet course ( Fig. 1 ). The nerve runs straightly along the foramen rotundum, and then turns laterally into the upper pterygopalatine fossa (PPF) and inferior orbital fissure before bending anteriorly into the orbit, through the infraorbital groove, where it gives rise to the zygomatic and the infraorbital nerves. The zygomatic nerve passes along the lateral orbital wall, exits the orbit laterally, and pierces the temporal fascia to supply the skin over the lateral/upper cheek and the temple.
The infraorbital nerve can be regarded as the terminal branch of V2. It reaches the infraorbital canal and leaves the orbit through the infraorbital foramen, giving off three terminal branches: the palpebral, labial, and nasal nerves. These nerves innervate the skin of the lower eyelid, lower cheek, upper lip and gingiva, and skin of the lateral side of the external nose.
The infraorbital nerve also provides the middle and anterosuperior alveolar nerves. The posterosuperior alveolar nerve branches separately from the main trunk of V2 at the PPF.
Within the PPF, two ganglionic branches spring from the maxillary nerve and connect V2 with the pterygopalatine ganglion. Parasympathetic, sympathetic, and sensory fibers enter the ganglion, although only the parasympathetic fibers synapse in the ganglion.
These preganglionic parasympathetic fibers originate from the brainstem (superior salivary nucleus), pass with the nervus intermedius of the facial nerve, and leave the facial canal of the temporal bone as the GSPN. The nerve runs on the anterior surface of the petrous apex. At the foramen lacerum the GSPN is joined by the deep petrosal nerve, carrying postganglionic sympathetic fibers from the sympathetic plexus on the internal carotid artery. They form the pterygoid (vidian) nerve, which passes through the pterygoid canal and ends in the pterygopalatine ganglion.
Because of this connection with the ganglion, the branches of the maxillary nerve arising from the pterygopalatine ganglion contain not only sensory fibers but also autonomic fibers, which are distributed to glands and blood vessels. Among these branches are the superior nasal and nasopalatine nerves, pharyngeal nerve, and greater and lesser palatine nerves.
The superior nasal and nasopalatine nerves leave the pterygopalatine fossa through the sphenopalatine foramen. They supply the mucosa of the lateral nasal fossa, the nasal septum, and the oral mucosa around the incisive canal of the hard palate. The pharyngeal (pterygopalatine) nerve passes through the pterygovaginal canal to supply the nasopharyngeal mucosa.
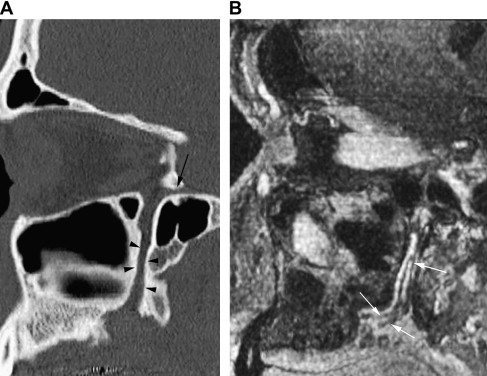
The greater and lesser palatine nerves run downward from the ganglion, through the greater palatine canal, and on the palate diverge, exiting at the greater and lesser palatine foramen, respectively ( Fig. 2 ). The greater palatine nerve innervates the posteroinferior lateral nasal wall mucosa and the hard palate mucosa (except around the incisive papilla). The lesser palatine nerve supplies the soft palate mucosa.
PNS along the peripheral branches of V2 is most frequently associated with neoplasms arising from the mucosa investing the oral cavity or oropharynx, particularly the hard and soft palate, and sinonasal tract. It can be observed in cutaneous malignancies arising from the facial skin innervated by V2 branches.
In several patients, PNS along V2 may be asymptomatic. Symptoms suggesting the involvement of peripheral branches of V2 or a more central extent include facial paresthesia or anesthesia in the V2 territory, atypical trigeminal neuropathy with facial pain, or concurrent impairment of other cranial nerves.
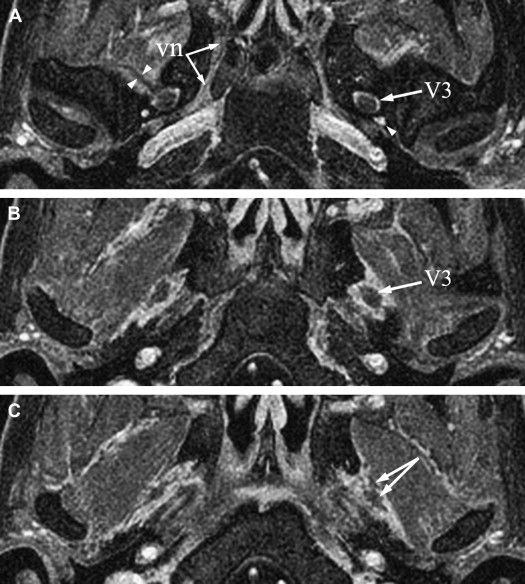
The mandibular nerve is the largest division of the trigeminal nerve. It leaves the skull base through the oval foramen and courses between the medial pterygoid muscle and its fascia (medially) and the lateral pterygoid muscle (laterally). A few millimeters below the foramen ovale, V3 divides into a small anterior trunk (mainly motor) and a large posterior trunk, mainly sensory ( Fig. 3 A–C).
The anterior division distributes the masseteric nerve, deep temporal nerves, nerve to the lateral pterygoid muscle (all are motor branches), and buccinator (buccal) nerve, which supplies sensory fibers for the mucosa and skin of the cheek. PNS along these branches may cause atrophy of corresponding masticator muscles. Involvement of these branches usually occurs with neoplasms arising from the masticator space, nasopharynx, or oropharynx, or through retrograde spread along the inferior alveolar nerve.
The posterior trunk of V3 divides into three nerves: the ATN, lingual nerve, and inferior alveolar nerve.
The ATN is mainly sensory but carries autonomic fibers for the parotid gland, derived from small branches connecting to the otic ganglion. The postganglionic parasympathetic fibers from the VII and IX nerves are carried to the otic ganglion by the lesser superficial petrosal nerve. The ATN is the first branch of the posterior division of V3. The nerve then splits into two roots that encircle the middle meningeal artery ( Fig. 3 D–F) and then merge again to continue as a short trunk, which runs close to the medial aspect of the temporomandibular joint. The ATN then pierces the parotid fascia and enters the retromandibular parotid gland, where it lies immediately above the bifurcation of the external carotid artery into the superficial temporal and internal maxillary arteries. Just posterior to the masseter muscle, two rami connect the ATN to the facial nerve. These connections provide sensory fibers for the zygomatic, buccal, and mandibular divisions of the facial nerve to the skin. Other branches of the ATN supply the temporomandibular joint and the skin of the anterosuperior portion of the auricle, the external acoustic meatus, and the temporal region.
Symptoms related to PNS along the ATN include periauricular pain and otalgia. The occurrence of facial weakness (suggesting VII nerve palsy), temporomandibular joint dysfunction, or pain, and V3 palsy indicate extensive tumor spread. Neoplasms arising from skin or parotid gland may follow the ATN neural pathway, sometimes with simultaneous spread along the facial nerve.
Although the lingual nerve is a sensory nerve, it also contains parasympathetic fibers because of its fusion with the chorda tympani branch of the facial nerve. The nerve runs beneath the lateral pterygoid muscle and then bends forward and downward in the space between the medial pterygoid muscle and the ramus of the mandible to reach the lingual alveolar ridge of the mandible. The nerve then courses along the floor of the mouth lateral to the hyoglossus muscle, twists twice around the submandibular gland duct, and enters the tongue behind the sublingual gland. The lingual nerve supplies the mucosa of the anterior two thirds of the tongue, the floor of the mouth, the lingual gingiva of the mandibular teeth, and the buccal mucosa. The chorda tympani provides parasympathetic fibers for the submandibular ganglion (which distributes postganglionic fibers to the sublingual and submandibular glands) and sensory fibers associated with taste for the anterior two thirds of the tongue. Dysgeusia, sensory impairment, and pain may be associated with lingual nerve invasion.
The inferior alveolar nerve is the third branch of the posterior trunk of V3. In addition to sensory fibers, it carries motor fibers for the mylohyoid muscle and the anterior belly of the digastric muscle (the mylohyoid nerve). The inferior alveolar nerve descends along the medial surface and inferior border of the lateral pterygoid muscle and then enters the mandibular foramen together with the inferior alveolar artery. The main distribution of the nerve is to the mandibular teeth. A cutaneous branch supplying the skin of the chin and inferior lip is the mental nerve. It exits the mandible through the mental foramen.
Involvement of the mental nerve can cause dysesthesia or numbness from the chin to the lower lip extending to the midline, often associated with anesthesia of the mucosal surface of the cheek, anterior gums, or lips. However, with alveolar nerve invasion up to the mandibular foramen, symptoms also are noted in the teeth and gums of the posterior ipsilateral mandible. Masticator space extent may cause denervation of the mylohyoid muscle and anterior belly of the digastric muscle.
The otic ganglion lies immediately below the foramen ovale on the medial surface of V3. It primarily supplies the parotid gland. Parasympathetic fibers (from the inferior salivary nucleus) reach the ganglion through the glossopharyngeal nerve, tympanic plexus in the middle ear, and lesser superficial petrosal nerve, which passes through the foramen ovale. Its sympathetic root consists of a filament from the plexus surrounding the middle meningeal artery. The sensory root is the ATN.
Facial Nerve
The facial nerve emerges from the groove between the medulla and pons. It has a large motor root and a smaller sensory root (nervus intermedius). The nerve crosses the cerebellopontine angle cistern with the vestibulocochlear nerve and runs through the internal auditory canal to reach the fundus. It then enters the facial canal, which it follows to the end at the stylomastoid foramen. On emerging from the stylomastoid foramen, the facial nerve courses downward, lateral to the styloid process and medial to the digastric muscle, to enter the parotid gland. At this level, the nerve divides into the temporofacial and cervicofacial branches, which show a variable intraglandular pattern of branching. From this parotid plexus usually emerge five branches for facial and cranial muscles (buccinator, orbicularis oculi, muscles of the upper and lower lips). The two segments of the facial nerve that are most likely to be involved in PNS are the descending portion of the intratemporal course of the nerve (the mastoid part) and the GSPN.
Neoplasms arising from or invading the parotid gland account for most PNS along the mastoid segment of the facial nerve. Primary tumors include adenoid cystic carcinoma, adenocarcinoma, mucoepidermoid carcinoma, and SCC. Among neoplasms secondarily invading the gland are cutaneous carcinoma and melanoma.
Further retrograde spread up to the geniculate ganglion and, less frequently, through the labyrinthine or intracanalicular segments is possible. Different degrees of facial palsy may accompany PNS.
The GSPN was described in the section discussing trigeminal nerve anatomy as a neural connection between the facial nerve and V2. Parasympathetic fibers travel by way of the GSPN and then the vidian nerve to reach the pterygopalatine ganglion and distribute to the mucosa through V2 peripheral branches. This path serves as a duplicate conduit for retrograde PNS by tumors that reach the pterygopalatine ganglion, the usual pathway being the maxillary nerve and Meckel’s cave. As a result, a hard palate adenoid cystic carcinoma spreading through the greater palatine nerve and reaching the pterygopalatine ganglion may subsequently follow either the V2 or the parasympathetic path (or both), ultimately extending into the temporal bone.
Hypoglossal Nerve
The hypoglossal nerve is a motor nerve supplying the musculature of the tongue. It originates from the medulla oblongata as 10 to 12 nerve roots that are fusing into 1 or 2 trunks piercing the dura mater and entering the hypoglossal canal of the occipital bone. The nerve emerges deep to the carotid sheath and runs downward close to the vagus nerve between the internal jugular vein and the internal carotid artery. It loops around the occipital artery, continues forward passing below the submandibular gland, lateral to the hyoglossus muscle, to be distributed to the muscles of the tongue.
Although PNS from tongue neoplasms has been described, the nerve is more frequently involved by nasopharyngeal carcinoma accessing the retrostyloid compartment of the parapharyngeal space.
Neural pathways relevant for perineural spread
In most cases the peripheral branches of the second (V2) or third division (V3) of the trigeminal nerve and the descending branch of the facial nerve serve as conduits for perineural tumor spread into the skull base.
Preexisting neural pathways, such as the vidian nerve, greater superficial petrosal nerve (GSPN), and auriculotemporal nerve (ATN), account for neoplastic dissemination from trigeminal branches to those of the facial nerve (and vice versa). Less frequently, the ophthalmic nerve (V1) or hypoglossal nerve are involved. PNS along other nerve routes has been reported in few patients. Perineural tumor spread is usually antegrade (toward the central nervous system), but retrograde spread may also occur.
Trigeminal Nerve
The trigeminal nerve is the largest of the cranial nerves, with a diameter of approximately 2.5 mm when measured a few millimeters from the entry point into the pons. The nerve exits the ventral surface of the pons and traverses the prepontine cistern. Once it reaches the petrous apex, the nerve runs through a defect of the dura and enters Meckel’s cave, which is located lateral to the cavernous sinus. Meckel’s cave is a trigeminal cistern filled with cerebrospinal fluid (CSF) and enclosed by two dural layers: the external layer, derived from the internal layer of cranial periosteum, and the internal layer, the true meningeal dura mater.
The sensory rootlets of the trigeminal nerve reach the concave gasserian ganglion, placed on the floor of Meckel’s cave. The ganglion has a semilunar shape. The concave surface (posteromedial) is outlined by the CSF. From the (opposite) convex surface, which blends with the dura of the floor of Meckel’s cave, originate the three branches of the trigeminal nerve. The ophthalmic nerve (V1) and the maxillary nerve (V2) are purely sensory, whereas the mandibular nerve (V3) has both sensory and motor functions.
The ophthalmic nerve divides into three branches (lacrimal, frontal, and nasociliary nerves) immediately before entering the superior orbital fissure. These nerves supply branches to the lacrimal gland and conjunctiva; to the skin of the eyelids, eyebrow, forehead, and nose; to the cornea, ciliary body, and iris; and to the mucosa of the nasal cavity.
Although PNS along V1 can occur with malignancies arising from the mucosa investing the nasoethmoid complex or from intraorbital neoplasms, it is generally caused by cutaneous malignant neoplasms (SCC or BCC, melanoma). In this setting, the two terminal branches of the frontal nerve are the most likely to be invaded: the supraorbital nerve (largest branch, runs between the orbital vault and the levator palpebrae superioris, leaves the orbit through the supraorbital foramen) and the supratrochlear nerve (runs medially to the pulley for the superior oblique muscle).
Numbness or pain in the forehead or upper eyelid skin and conjunctiva are associated with involvement of the two terminal branches of the frontal nerve.
The maxillary nerve shows a particular bayonet course ( Fig. 1 ). The nerve runs straightly along the foramen rotundum, and then turns laterally into the upper pterygopalatine fossa (PPF) and inferior orbital fissure before bending anteriorly into the orbit, through the infraorbital groove, where it gives rise to the zygomatic and the infraorbital nerves. The zygomatic nerve passes along the lateral orbital wall, exits the orbit laterally, and pierces the temporal fascia to supply the skin over the lateral/upper cheek and the temple.
The infraorbital nerve can be regarded as the terminal branch of V2. It reaches the infraorbital canal and leaves the orbit through the infraorbital foramen, giving off three terminal branches: the palpebral, labial, and nasal nerves. These nerves innervate the skin of the lower eyelid, lower cheek, upper lip and gingiva, and skin of the lateral side of the external nose.
The infraorbital nerve also provides the middle and anterosuperior alveolar nerves. The posterosuperior alveolar nerve branches separately from the main trunk of V2 at the PPF.
Within the PPF, two ganglionic branches spring from the maxillary nerve and connect V2 with the pterygopalatine ganglion. Parasympathetic, sympathetic, and sensory fibers enter the ganglion, although only the parasympathetic fibers synapse in the ganglion.
These preganglionic parasympathetic fibers originate from the brainstem (superior salivary nucleus), pass with the nervus intermedius of the facial nerve, and leave the facial canal of the temporal bone as the GSPN. The nerve runs on the anterior surface of the petrous apex. At the foramen lacerum the GSPN is joined by the deep petrosal nerve, carrying postganglionic sympathetic fibers from the sympathetic plexus on the internal carotid artery. They form the pterygoid (vidian) nerve, which passes through the pterygoid canal and ends in the pterygopalatine ganglion.
Because of this connection with the ganglion, the branches of the maxillary nerve arising from the pterygopalatine ganglion contain not only sensory fibers but also autonomic fibers, which are distributed to glands and blood vessels. Among these branches are the superior nasal and nasopalatine nerves, pharyngeal nerve, and greater and lesser palatine nerves.
The superior nasal and nasopalatine nerves leave the pterygopalatine fossa through the sphenopalatine foramen. They supply the mucosa of the lateral nasal fossa, the nasal septum, and the oral mucosa around the incisive canal of the hard palate. The pharyngeal (pterygopalatine) nerve passes through the pterygovaginal canal to supply the nasopharyngeal mucosa.
The greater and lesser palatine nerves run downward from the ganglion, through the greater palatine canal, and on the palate diverge, exiting at the greater and lesser palatine foramen, respectively ( Fig. 2 ). The greater palatine nerve innervates the posteroinferior lateral nasal wall mucosa and the hard palate mucosa (except around the incisive papilla). The lesser palatine nerve supplies the soft palate mucosa.