Imaging of the vestibulocochlear nerve has evolved dramatically over the past few decades. The imaging specialist now is involved in the diagnosis of far more diagnostic entities than ever before. With this increased involvement comes the responsibility to increase collective knowledge regarding the pathophysiology of these diagnostic entities. This article is organized in a conventional way and covers congenital deformity of the internal auditory canal, neoplastic and pseudoneoplastic lesions, with special detailed emphasis on schwannoma of the eight cranial nerves (acoustic neuroma), nonneoplastic IAC/CPA pathology, including vascular loops, and numerous additional differential diagnostic entities, with particular emphasis on non-neoplastic meningeal disease. Lesions of the auditory pathway and an overview of cochlear implant surgery are also included in this discussion.
This article covers congenital deformity of the internal auditory canal (IAC), neoplastic and pseudoneoplastic lesions with special detailed emphasis on schwannoma of the eight cranial nerve (acoustic neuroma), non-neoplastic IAC/cerebellopontine angle mass (CPA) pathology, including vascular loops and numerous additional differential diagnostic entities with particular emphasis on non-neoplastic meningeal disease.
Congenital deformity of the internal auditory canal
The caliber of the internal auditory canal (IAC) depends upon the development of the vestibulocochlear nerve. This is consistent with findings noted in the evaluation of the other skull base foramina, virtually all of which develop in response to the presence of the regional nerve or artery. As such, congenital vestibulocochlear nerve deficiency commonly is associated with a hypoplastic IAC containing only the facial nerve ( Fig. 1 ). Although measurements are somewhat arbitrary, the IAC is considered hypoplastic when less than 2 mm in diameter. This usually is appreciated best upon examination in the axial and coronal plane; however, high-resolution thin-section oblique sagittal T2W MR imaging not only allows demonstration of this small canal but also depicts to best advantage absence/hypoplasia of the vestibulocochlear nerve ( Fig. 2 ). The cochlear nerve is defined as hypoplastic when similar in size or smaller than either of the vestibular nerves.
Cochlear nerve deficiency in the presence of a normal-sized internal auditory canal implies that it is acquired after formation of the IAC. This has been observed most commonly in patients who have labyrinthitis ossificans ( Fig. 3 ).
Abnormality of vestibulocochlear nerve caliber has crucial implications for patients requiring cochlear implantation. Absence of the cochlear nerve is the only complete contraindication to this procedure. In addition to the IAC caliber, the imaging specialist must evaluate the status of the cochlear nerve aperture at the fundus of the IAC. Stenosis of this aperture consistently is associated with cochlear nerve deficiency also ( Fig. 1 B). Additional considerations regarding cochlear implantation are discussed later in this article.
Narrowing of the IAC may occur secondary to exostoses or osteomas. These bony lesions are rare, but they may clinically mimic vestibular schwannomas by compression of the vestibulocochlear nerve. Hearing loss and vestibular symptoms including dizziness and vertigo may improve after surgical removal of the bony lesion. Compromise of the IAC also may occur as a complication of several of the otodystrophies, including Paget’s disease and fibrous dysplasia.
A large IAC (greater than 9mm) may be incidental. Bilateral IAC enlargement is seen with neurofibromatosis (NF)-2 secondary to bilateral acoustic tumors. NF also may result in IAC widening because of dural ectasia without neoplasm.
The large IAC also has other important implications. Special attention to the fundus (lateralmost aspect) of the canal may reveal an unusually wide neural aperture leading to the cochlea or vestibule. This implies increased pressure within the perilymphatic space and predisposes to abnormal flow of perilymph (gusher) upon stapes manipulation. This can occur with various congenital inner ear malformations but is a classic manifestation of x-linked progressive mixed deafness (XLPMD) ( Fig. 4 ). These male patients present with mixed hearing loss possibly masquerading as otosclerosis clinically. If the deformity is recognized before middle ear exploration, the imaging specialist can provide a potentially life-saving service.
Neoplastic and pseudoneoplastic
The differential diagnosis of the IAC/cerebellopontine angle mass (CPA) is fairly clear-cut in that there is universal agreement regarding the four most common entities. Schwannoma of the eighth cranial nerve (acoustic neuroma) is the most common, followed by meningioma, arachnoid cyst, and epidermoid (in that order). The next several paragraphs summarize the most important clinical and imaging findings of these entities. Subsequent paragraphs will focus on the less common diagnostic entities.
Schwannoma of the Eighth Cranial Nerve (Acoustic Neuroma)
Overview
Schwannoma of the eighth cranial nerve (acoustic neuroma) is by far the most common CPA neoplasm. They are noncalcifying, slow-growing, well-encapsulated lesions of midlife that account for 6% to 10% of all intracranial tumors and 60% to 90% of CPA tumors. They are somewhat more common and tend to be larger in women. There are strong indications that pregnancy may accelerate the growth of this tumor. Schwannomas most commonly present as a combined IAC/CPA lesion; however, they may be entirely intracanalicular in nature. Interestingly, this latter subgroup of lesions is more common in men. Schwannomas arising from the extracanalicular portion of the nerve may involve only the CPA cistern, sparing the IAC and masquerade as meningioma, metastasis, or exophytic brainstem tumor. Intralabyrinthine schwannomas are rare and will be discussed in detail later.
Clinical issues
Eighth nerve schwannomas typically present with progressive unilateral high-frequency retrocochlear sensorineural hearing loss, presumably resulting from compression/infiltration of auditory (cochlear) nerve fibers. This hearing loss is often manifested clinically by disproportionately deficient speech discrimination (compared with pure tone loss) as measured by brainstem electric response audiometry. Sudden worsening of hearing deficit occurs commonly (26%) and presumably is associated with occlusion of the internal auditory branch of the anterior inferior cerebellar artery (AICA). A small number of patients have normal hearing (or symmetric loss). The clinician must maintain suspicion when nonauditory symptoms predominate such as tinnitus or vertigo. Tinnitus in this context is typically high-pitched and unilateral (tumor side). Vertigo, an episodic illusion of motion, and horizontal nystagmus, are manifestations of peripheral vestibular dysfunctions that typically occur early in the course of tumor development, possibly because of disruption of vascular supply. Disequilibrium, a continuous sense of instability, occurs with larger lesions, possibly because of deafferentation (compromise of sensory nerve impulses caused by injury of sensory nerve fibers). The presence of vertical nystagmus often implies a large tumor with brainstem compression. The facial nerve may undergo substantial torsion without losing its functional integrity; therefore associated facial palsy is unusual. Recall that although the facial nerve is predominately motor, there is a sensory component that supplies a portion of the external auditory canal and pinna. Diminished sensation in this distribution is referred to as Hitselberger’s sign. Large lesions are more likely to cause fifth nerve symptoms (facial numbness, diminished corneal reflex). Facial pain, presumably caused by vascular impingement on the trigeminal root entry zone, is uncommon but not rare. Intention tremor and ataxia result from cerebellar compression.
Despite the progressive nature of the hearing deficit, eighth nerve schwannomas arise from the vestibular rather than the cochlear portion of the nerve in approximately 85% of these patients. The superior and inferior vestibular nerves are equally likely to be the site of origin.
Imaging issues
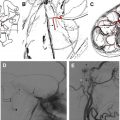
The internal auditory canals have a strong tendency for symmetry, similar to the optic canals. This was the historical basis for schwannoma diagnosis with plain film and polytomography, modalities that required precise comparative measurements and identification of subtle erosive change. With the development of CT, direct visualization of the CPA component of the tumor could be accomplished, and it simultaneously was discovered that a significant percentage of normal individuals have asymmetric IACs. Despite advancements in CT, the direct diagnosis of intracanalicular lesions remained difficult until the development of gas CT cisternography. This technique required the placement of a small amount (2 to 3 mL) of intrathecal air or CO 2 by means of lumbar puncture. The patient was imaged in the decubitus position with the affected ear up. Using this technique, very small lesions first were diagnosed, and an appreciation of CPA/IAC neurovascular anatomy was mandated. The emergence of thin-section gadolinium-enhanced T1 weighted MR imaging sequences revolutionized diagnosis, as very small lesions easily could be diagnosed less invasively, and larger lesions were characterized more readily ( Figs. 5 and 6 ). More recently, thin-section T2 weighted fast-spin-echo (FSE) and gradient-echo techniques have been developed that provide highly sensitive screening for these lesions without the use of gadolinium. FSE images have the advantage of far less tendency for magnetic susceptibility artifact, and resolution is increased further by the use of phased array receiver coils. Cerebrospinal fluid (CSF) provides an excellent contrast in this context, as virtually all schwannomas are of lower T2 signal intensity. This contrast is more apparent with longer TR (4000 to 5000 ms). Such long repetition times (TR) are practical because of the decreased acquisition time resulting from the long echo trains inherent to this technique.
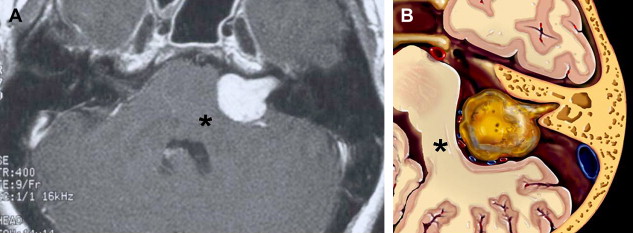
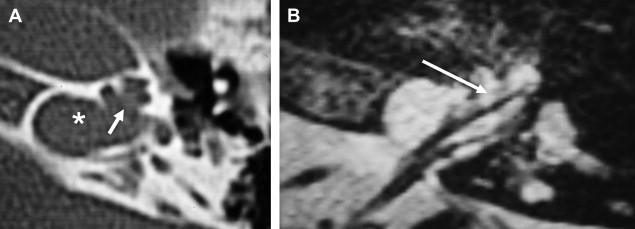
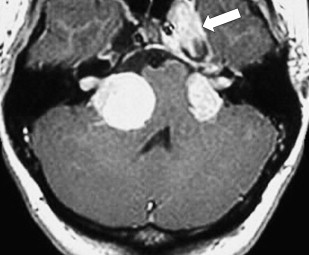
There is some disagreement regarding the precise site of origination of eighth nerve schwannomas. It has been postulated that they arise in most cases at the transition zone between the myelin produced by the oligodendroglia (central myelin) and that produced by Schwann’s cells (peripheral myelin). This neuroglial/neurilemmal junction, referred to as the Obersteiner-Redlich zone, occurs most commonly at the Scarpa’s (vestibular) ganglion typically (but not invariably) located at or in close proximity to the meatus (porus) of the IAC. Others have suggested that these tumors originate from Scarpa’s ganglion because this is the area of the highest concentration of Schwann cells. Extracanalicular lesions invariably arise in close proximity to the meatus. No vestibular Schwannoma ever has been diagnosed at the brainstem root entry zone or even within the medial half of the cisternal segment of the nerve. Schwannomas only uncommonly arise within the lateral IAC (fundus), and therefore, imaging diagnosis of intracanalicular lesions in this specific location must be viewed with suspicion ( Fig. 7 ). False-positive examinations are associated with the presence of relatively faint fundal enhancement. Nonneoplastic conditions encountered during surgical exploration of these lesions has lead to speculation that perhaps a wait-and-see approach should be advocated in this circumstance, with reimaging in 6 months. Disappearance of this faint enhancement has been documented in several patients.
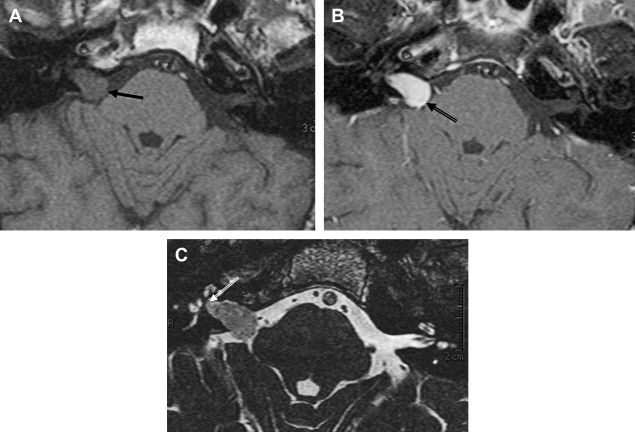
Histologic examination of schwannomas reveal compact Antoni type A tissue and loose textured, often cystic, Antoni type B tissue. The predominance of the Antoni type A variety helps explain this tendency toward T2 hyposignal relative to CSF. As schwannomas enlarge, regions of internal necrosis/cyst formation may result in a heterogeneous appearance. These are of higher signal intensity than CSF presumably because of the presence of hemorrhagic byproducts, necrotic material, or colloid-rich fluid. These larger lesions have a predominance of Antoni type B cells. In addition to intratumoral cysts, extramural (arachnoid) cysts also are associated with large lesions presumably secondary to elevation and deformation of the leptomeninges, which results in the formation of peritumoral adhesions, thereby creating a pseudoduplication of the arachnoid, trapping fluid between the leptomeninges and the mass. Extramural cysts also are associated with meningiomas by means of a similar mechanism. Intratumoral hemorrhage is rare and associated with head injury or vigorous physical exertion. Both intratumoral hemorrhage and cystic expansion may result in a rapid increase in tumor volume. These patients often develop a sudden severe worsening of symptomatology ( Fig. 8 ).
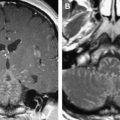
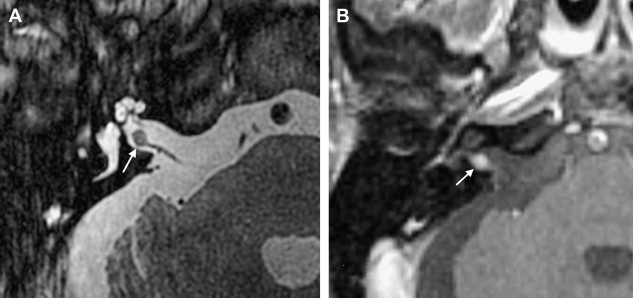
The reader should be aware that diminished signal intensity in the vestibule on T2-weighted images has been demonstrated in patients who have cerebellopontine angle/IAC schwannoma (acoustic neurinoma), but not in patients who have CPA meningioma. This decreased signal intensity may result from increased protein concentration in the perilymph associated with acoustic tumors. Some patients who have vestibular schwannoma larger than 2 cm in diameter demonstrate a tiny area of hyperintensity in the dorsal brain stem on T2 weighted images at the lateral angle of the fourth ventricle floor. This may represent degeneration of the vestibular nucleus. This should not be confused with infarction.
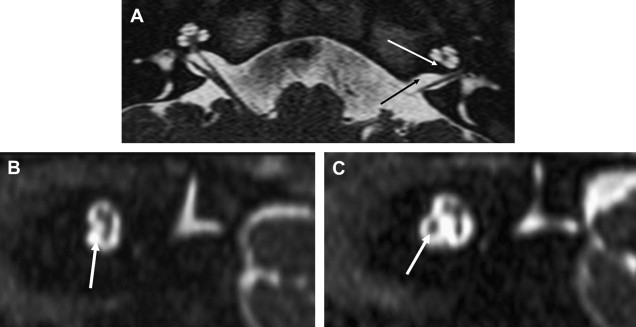
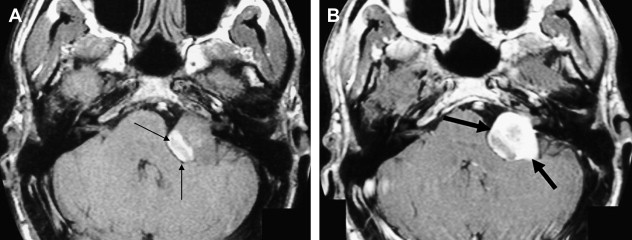
Eighth nerve schwannomas are typically benign and slow growing. Malignant degeneration is rare and usually associated with NF 1. These patients have an increased incidence of schwannomas; however, fewer than 5% have eighth nerve schwannoma. By contrast, the identification of bilateral eighth nerve schwannomas is the cornerstone of imaging diagnosis of the far less common NF 2, so-called central NF ( Fig. 9 ). These patients typically also have multiple meningiomas, neurofibromas, and glial tumors. Eighth nerve lesions may develop at a young age and MR imaging screening is recommended. The gene responsible for this neoplasm has been isolated to the long arm of chromosome 22.
Surgical issues
The collaboration of the imaging specialist and the surgical team is required in the choice of a definitive surgical procedure when an eighth nerve tumor is diagnosed. Three major approaches are employed: translabyrinthine (TL), retrosigmoid (RS), and middle fossa (MF). Factors influencing the decision include the depth of tumor extension into the lateralmost IAC (fundal involvement), the size of a CPA component, and the prognosis for hearing conservation.
If there is no serviceable hearing, or the prognosis for acceptable hearing is dim, the TL approach is most desirable, as surgical morbidity is reduced greatly. The TL approach is the most direct and allows for identification of the facial nerve and easy access to the fundus; it is required when an intralabyrinthine component is present. Despite common misconception, the surgical exposure necessary for removal of large CPA components can be accomplished in experienced hands.
Hearing conservation requires either an MF or RS approach. In this context, the imaging findings become especially crucial. The presence of a significant CPA component (greater than 1.5 cm) precludes the MF technique because of inadequate surgical exposure. The presence of a fundal component precludes the RS technique, because a portion of the labyrinth would have to be removed to expose the lateral third of the IAC (see Fig. 6 ). There are strong indications that FSE magnetic resonance sequences using T2 weighting are more sensitive to the presence of fundal tumor, because bright CSF provides better contrast than the dark CSF visualized on gadolinium MR imaging (T1 weighting).
The MF approach is ideal for fundus lesions. The MF technique affords the greatest likelihood of hearing conservation but does so at the expense of the need for greater facial nerve manipulation, as the anterosuperior location of the nerve within the IAC places the nerve between the surgeon and the tumor. This is especially true when the neoplasm arises from the inferior (IVN) instead of the superior vestibular nerve (SVN).
The RS approach is another example of potential hearing preservation surgery and affords the exposure to remove lesions with large CPA components. A subocciptal craniotomy medial to the sigmoid sinus is followed by cerebellar retraction and removal of the posterior lip of the IAC.
CSF leak can occur with any of the three surgical approaches, although this complication is more common with the retrosigmoid approach and with larger tumors. Translabyrinthine surgery can result in a middle ear CSF fistula that drains through the nose through the eustachian tube, resulting in rhinorrhea.
Loss of inner ear signal on T2WI correlates with loss of hearing in patients who have undergone surgery for CPA and IAC vestibular schwannoma, perhaps reflecting developing inner ear fibrous changes or ossification. Normal cochlear T2 signal is associated with hearing preservation in this circumstance.
Radiosurgery (gamma knife treatment [GKT]) may be recommended, and the imaging specialist should be familiar with this procedure and the post-treatment appearance. This method has been helpful for patients who for other health reasons might be considered poor operative candidates. Often, there is paradoxically more heterogeneous enhancement in the first 6 to 12 months after GKT, as the center of the lesion undergoes cell death and fibrosis. Temporary enlargement has been reported within 2 years following treatment; therefore lesions larger than 3 cm and those with significant brainstem compression are excluded from radiosurgery.
Postoperative imaging
The imaging diagnosis of recurrent schwannoma may be challenging. Postoperative intracanalicular enhancement with gadolinium is very common. Such enhancement may be caused by recurrent tumor, adhesions, or meningeal irritation. Temporalis muscle and fascia, commonly used to seal the bony IAC defect created with the MF approach, results in a globular enhancement pattern. A linear enhancement pattern within the postoperative IAC is another predictable postoperative appearance. Other postoperative features include T1 shortening in the labyrinth, possibly representing methemoglobin or elevated protein concentration, and the presence of new labyrinthine enhancement, which may be caused by labyrinthitis or granulation tissue. Intracanalicular enhancement also may occur within the facial nerve as the result of surgical manipulation of this structure and must not be confused with tumor recurrence.
Postoperative MR imaging study performed within 3 days after surgery may be the most helpful asset for determining the presence of residual disease and serves as a baseline study for subsequent evaluation for recurrence. Follow-up studies that demonstrate a change in the enhancement pattern should be viewed with suspicion, especially when this enhancement becomes nodular. The retrosigmoid approach has the greatest risk of unintentional residual tumor in the lateral (fundal) aspect of the IAC, and this area should be searched carefully with postoperative scans.
Intralabyrinthine schwannoma
Intralabyrinthine schwannomas (ILS) arise from distal branches of the cochlear, superior vestibular, or inferior vestibular nerves. They may originate from vestibular nerve fibers of any of the cristae or maculae within the vestibule or nerve fibers of the cochlear nerve within the cochlea Box 1 .
Localized to labyrinth
Intracochlear
Intravestibular
Intravestibulocochlear
Internal auditory canal involvement
Transmodiolar (cochlea and IAC)
Transmacular (vestibule and IAC)
Middle ear involvement
Transotic (ME, labyrinth and IAC)
Tympanolabyrinthine (ME and labyrinth)
Data from Tieleman A, Casselman JW, Somers TH, et al. Imaging of intralabyrinthine schwannomas: a retrospective study of 52 cases with emphasis on lesion growth. AJNR Am J Neuroradiol 2008 [Epub ahead of print].
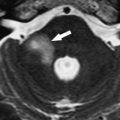
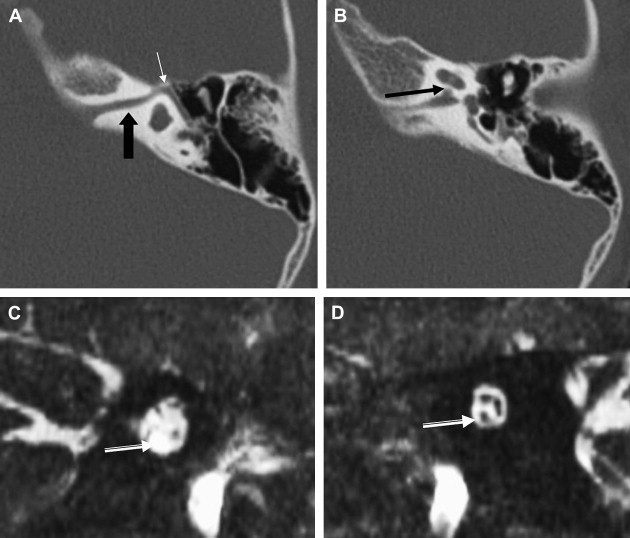
ILS is rare, although recently a larger series and a more frequent occurrence has been reported. They are somewhat more common in patients who have NF-2, but most cases are sporadic. Lesions that are limited to the labyrinth are classified as intracochlear, intravestibular, and intravestibulocochlear. Lesions that also involve the IAC are described as transmodiolar (cochlea and IAC) or transmacular (vestibule and IAC) ( Figs. 10 and 11 ). Lesions that also involve the middle ear are described as transotic (ME, labyrinth, and IAC) or tympanolabyrinthine (ME and labyrinth). All patients report sensorineural hearing loss that is of sudden onset in perhaps one third of cases. ILS is histologically identical to IAC counterparts and occasionally presents with Meniere-type symptoms, although recent data indicate that presentation is typically indistinguishable from that which occurs with typical eighth nerve schwannoma. A loss of conductive hearing is not uncommon and results from blockage of sound wave movement through inner ear fluid.
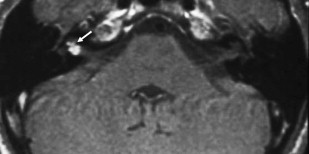
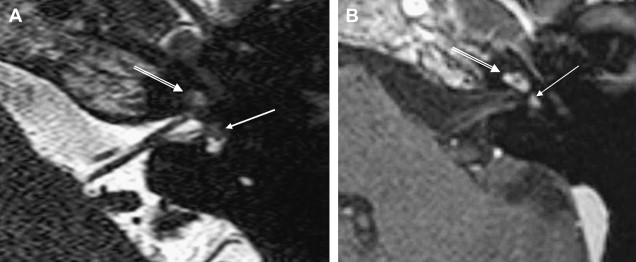
Prior to the development of modern imaging methods, diagnosis usually was made during surgical labyrinthine ablation. Currently, the mainstays of diagnosis are thin-section Gadolinium-enhanced T1 weighted images that demonstrate a focal area of intense localized enhancement within the labyrinth ( Fig. 12 ). This appearance is usually quite different from the faint, diffuse enhancement seen with labyrinthitis. Most cases will be discovered during routine evaluation of the “rule out acoustic” patient. The observer is encouraged to avoid IAC tunnel vision and meticulously examine the fluid-filled spaces of the labyrinth also. ILS also can be visualized with carefully preformed thin-section FSE T2WI as regions of hyposignal within the normally fluid intensity (bright) labyrinth (see Figs. 11 and 12 ).
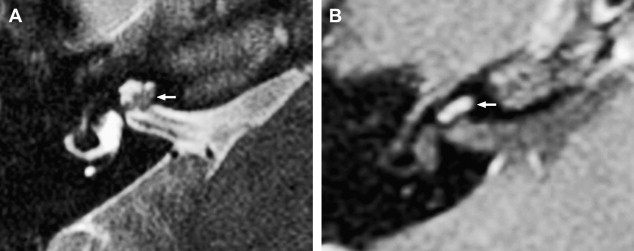
The reader should be aware that nontumorous labyrinthine enhancement may occur secondary to a fundal IAC lesion likely caused by venous engorgement and inflammation. This is referred to as IAC block.
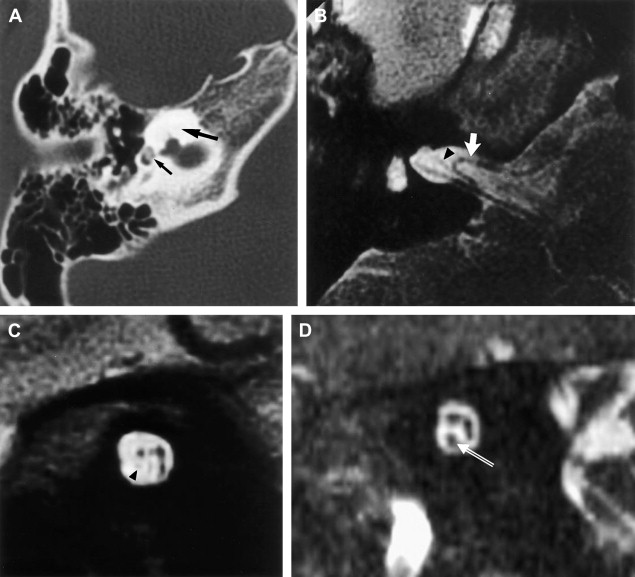
Intralabyrinthine neoplasms other than schwannoma are even more rare. Intralabyrinthine meningiomas are also intensely enhancing lesions. IAC meningioma likely arises from arachnoid granulations located along the dural lining of the neural foramina. They may spread to the labyrinth, resulting in intense enhancement, often with ossification and demineralization reminiscent of otospongiosis. The presence of associated ossification may be the key to diagnosis, although an atypically positioned ossified hemangioma also must be considered as a differential diagnostic possibility under this circumstance.
Intravestibular lipomas have been reported manifest by precontrast T1 hypersignal, which disappears with fat suppression techniques. Neural transport of the meninx primitiva along the developing eighth nerve has been proposed as an etiology.
Other Cerebellopontine Angle Mass Schwannomas
Vestibular schwannoma is clearly the most common intracranial nerve sheath tumor; however, schwannomas arising from CN5 to CN12 may result in a CPA mass. The nerve of origin of most CPA schwannomas often can be determined by the clinical symptomatology and specific imaging epicenter. The facial nerve schwannoma (FS) arising in the IAC or CPA may cause the most difficulty with differentiation from vestibular schwannoma. The key differentiating feature is the presence of an enhancing component extending along the labyrinthine segment of the facial nerve to the geniculate ganglion. At CT, bone windows may reveal enlargement of the facial nerve canal.
Meningiomas
Meningiomas are extra-axial neoplastic lesions arising from arachnoidal cap cells. Approximately 10% of all intracranial meningiomas arise in the posterior fossa. They are the second most common CPA tumor, constituting approximately 10% of masses in this location. CPA meningiomas usually arise from the posterior petrous surface or the underside of the tentorium. Intense enhancement is the rule. Relative T2 hyposignal is characteristic of many of these lesions, especially the transitional and fibroblastic varieties ( Fig. 13 ).
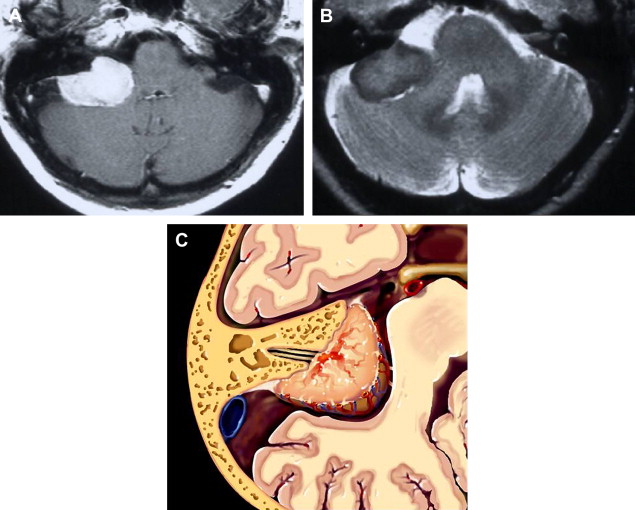
Differentiation from acoustic tumor is based primarily on remoteness of the epicenter from the porus of the IAC and the presence of a broad base. Occasionally, meningiomas will masquerade as acoustic tumors when the epicenter is indeed at the meatus. Extension of tumor into the IAC is not rare in this context. The presence of a dural tail once was thought to be pathognomonic of meningioma and represent tumor extending along the adjacent dura; however, not only have histologic studies proven that the tail is often merely an inflammatory response, but numerous neoplasms and even non-neoplastic entities have been discovered to exhibit this nonspecific imaging characteristic. Nonetheless, in some circumstances, this remains a somewhat useful imaging finding.
Up to 75% of CPA meningiomas present with hearing loss similar to acoustic tumors. These tumors tend to present in middle-aged to elderly patients, especially women, and they are rare in young patients in the absence of NF2. As with meningiomas elsewhere, there is a tendency toward multiplicity, and the current and follow-up scans should be evaluated carefully for additional lesions.
As with meningiomas found elsewhere, they tend to be hyperdense to brain at noncontrast CT imaging with homogeneous contrast enhancement. Focal areas of calcification are appreciated best before contrast and evident in up to 25% of cases.
In contradistinction to schwannoma, necrosis and cystic degeneration are uncommon with meningiomas. Rarely, meningiomas may originate within the internal auditory canal, or even within the labyrinth. In these latter circumstances, differentiation from schwannoma is even more difficult. IAC meningiomas may spread to the labyrinth, often with ossification and demineralization reminiscent of otospongiosis. Relative T2 hypointensity is a helpful imaging finding, suggesting dense cellularity, and supports a diagnosis of tumor rather than reactive enhancement.
Epidermoid
An epidermoid is an ectodermal inclusion that develops during neural tube closure in the third to fifth week of embryogenesis and comprises .2% to 1.8% of primary intracranial masses. Histologically identical to congenital cholesteatoma, they consist of a sac of exfoliated keratin lined by stratified squamous epithelium. They grow very slowly by means of desquamation and therefore often do not present until adulthood despite their congenital etiology. Epidermoids may become quite large before becoming symptomatic, because they have a strong tendency to grow along paths of least resistance, and, as such, conform to the surface of the brain tending to surround (encase) normal neural and vascular structures rather than displace or invade them. This tendency also often makes complete surgical resection difficult.
The CPA is the most common location for the intracranial epidermoid (40% to 50%). They are the third most common overall CPA/IAC mass (3% to 7%) (after acoustic schwannoma and meningioma).
At CT, most epidermoids are CSF density and do not enhance. Calcification is present in 10% to 25% of cases. An occasional lesion will have a faintly enhancing border or flecks of calcification.
Most epidermoids are isointense to CSF on both T1 and T2 weighted magnetic resonance images. They exhibit this signal, because the cholesterol in epidermoid tumors is in the solid/crystalline state rather than the liquid state. The latter would result in bright T1 signal. The adamantinomatous craniopharyngioma typically contains this type of cholesterol; epidermoids rarely do. Most epidermoid cysts do not enhance, although some minimal rim enhancement occurs in approximately 25% of cases. Malignant transformation of an epidermoid (squamous cell carcinoma) is rare but should be considered if follow-up scans demonstrate a significant increase in the amount of enhancement ( Box 2 ).
Epidermoid
Internal septations
Lamellated appearance
Encase
Insinuate
Higher signal than csf on flair
Diffusion weighted imaging (DWI): restricted diffusion-marked hyperintensity
Arachnoid cyst
Homogeneous
Smooth contour
Displace
Expand
Identical to CSF on flair
DWI: unrestricted diffusion—identical to CSF
Often, MR imaging is focused on differentiation from arachnoid cyst. Careful MR imaging examination commonly will reveal multiple internal septations within the epidermoid that are not present with arachnoid cyst. Furthermore, many epidermoids often have a lamellated appearance because of surface desquamation. This lamellation as well as the tendency to have internal septations and to insinuate rather than displace are far more characteristic of epidermoid. They do not suppress completely on fluid-attenuated inversion recovery imaging and therefore are higher in signal intensity than CSF. State-of-the-art differentiation of epidermoids from arachnoid cysts requires DWI ( Fig. 14 ). Epidermoids are bright on diffusion imaging because of low water diffusability (low apparent diffusion coefficient, or ADC). This restricted diffusion results in marked hyperintensity, quite different from arachnoid cysts. DWI signal also is useful for detecting postoperative residual epidermoid.
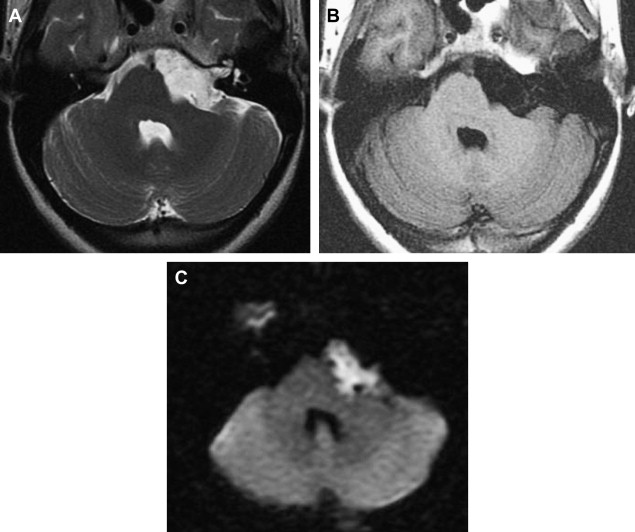
The rare “white epidermoid” is an interesting variant that is hyperdense at CT, hyperintense on T1WI, and hypointense on T2W. This may be explained by high protein content, hemorrhage or the presence of saponified keratinized debris, and as indicated previously, cholesterol in the liquid state.
Like epidermoid cysts, dermoid cysts are congenital ectodermal inclusions but are extremely rare, far less common than epidermoid cysts. Growth can lead to rupture of the cyst, causing a chemical meningitis. A common misconception is that dermoid cysts arise from both ectodermal and mesodermal elements. Their origin is entirely ectodermal. Imaging findings vary. Unruptured cysts are hyperintense, similar to fat on T1 weighted images. The masses have heterogeneous signal intensity on T2-weighted magnetic resonance images and vary from hypo- to hyperintense. Fat-like leptomeningeal and intraventricular deposits are diagnostic of a ruptured dermoid cyst.
Arachnoid Cysts
Arachnoid cysts are benign space-occupying lesions containing CSF. They reside entirely within the subarachnoid space but are surrounded by a thin membrane. They are typically unilocular, smoothly marginated, and expansile; they do not communicate with the ventricular system.
The precise mechanism for arachnoid cyst development is unknown. These lesions may develop through failure of embryonic meninges to merge or they may occur secondary to “splitting” of the developing arachnoid. Arachnoid cysts are generally stable over time. The cyst wall is lined by flattened arachnoid cells rather than epithelium.
Most arachnoid cysts are supratentorial. Fifty percent to 60% are found in the middle cranial fossa, anterior to the temporal lobes. Approximately 10% occur in the posterior fossa, most commonly in the CPA.
Arachnoid cysts are well demarcated extra-axial lesions that can displace adjacent structures. There is no identifiable internal architecture. They are isodense to CSF at CT and isointense to CSF on all magnetic resonance sequences. They do not enhance with either modality ( Fig. 15 ). Occasionally, hemorrhage, high protein content, or lack of flow within the cyst may complicate the magnetic resonance appearance. As discussed previously, the most difficult lesion to distinguish from the arachnoid cyst is epidermoid.
Meningeal Metastases
Cerebellopontine angle metastatic disease can present as a distinct extra-axial mass mimicking schwannoma/meningioma or as meningeal metastases associated with diffuse seeding of the CSF ( Fig. 16 ). Meningeal metastases is referred to by several ambiguous terms including leptomeningeal carcinomatosis, meningeal carcinomatosis, and carcinomatous meningitis. These terms are inexact, as these neoplasms are not always carcinomas. Additionally, they often involve the pachymeninges and the leptomeninges and may not contain an inflammatory (-itis) component. Meningeal metastases from solid tumors (ie, breast) and lymphoproliferative malignancy (ie, lymphoma) involve both the pachymeninges and the leptomeninges. Seeding from primary central nervous system (CNS) tumors involve only the leptomeninges. Leptomeningeal neoplasm may be diffuse or discrete and nodular. Nodular disease may masquerade as a primary neoplasm such as schwannoma or meningioma. Involvement of multiple cranial nerves is common. In this context, both facial nerve dysfunction and vertigo/hearing loss may result from CPA/IAC dissemination. A new diagnosis of NF2 in an adult patient should be considered suspect even when bilateral CPA/IAC lesions are diagnosed, and a thorough search should be performed for other intracranial metastases and the CSF sampled for malignant cells. Metastatic melanoma has a predilection for the leptomeninges and as such distinctively involve the IAC/CPA. Amelanotic lesions appear identical to schwannoma; however, melanotic lesions are hyperintense on precontrast T1WI, resulting in a confusing imaging picture suggestive of lipomatous infiltratration. There is no fat suppression, which aids in diagnosis.
Lipoma and Mimics
Lipomas are composed of mature adipose tissue, which is thought to arise from aberrant differentiation of the meninx primitiva. They are mesodermal inclusions, distinguishing them from epidermoid and dermoid, which are ectodermal in nature. They may be found in the CPA, IAC, or even more rarely, within the vestibule. Intracanalicular lipomas, despite their rarity, mandate routine precontrast thin-section T1 weighted images as part of a complete IAC examination.
At CT, lipomas are of fatty density and do not enhance. At MR imaging, they follow the signal intensity of fat, with suppression of signal on fat saturation images ( Fig. 17 ).