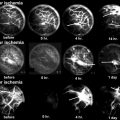
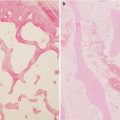
Fig. 18.1
Hematopoietic cells are depleted, and the marrow space is filled with large round or ovoid fat cells. Lacunae are filled with viable osteocytes (Arlet and Durroux Type 1 lesion) (hematoxylin and eosin stain, original magnification ×100)
This increase in fat cell volume leads to an intraosseous hypertension in the proximal femur [6, 8]. The intraosseous hypertension can cause venous sinusoidal compression, impaired arterial flow, and microvascular coagulation in the proximal femur [6].
A decrease of intramedullary blood flow induces a conversion of hematopoietic marrow to fatty marrow probably because fatty marrow can survive even with a limited vascular supply relative to hematopoietic marrow [33, 34].
Thus, marrow ischemia and fat marrow conversion mutually interact forming a vicious cycle. Regions composed of fatty marrow have a stronger predilection for ON than do areas of hematopoietic marrow. The femoral head, one of the first places to convert to fatty marrow, is at risk for ON [33, 34].
In Gaucher disease, a fatty substance “glycosphingolipid” is stored in marrow cells, which leads to an intraosseous hypertension [35].
18.4.2 Marrow Necrosis
Intravascular coagulation leads to an acute ischemia in the femoral head.
The hematopoietic cells are most sensitive and are the first to die after the ischemia, usually within 12 h [39]. The marrow fat cells die, and their nucleus disappears from the second day of ischemia [40]. Thus, the earliest microscopic signs indicative of bone ischemia are seen in the marrow spaces. The hematopoietic cells disappear, and there is loss of nuclear staining of marrow fat cells. The fatty marrow becomes necrotic (Arlet and Durroux Type 2 lesion) (Fig. 18.2). Necrotic lesions are scattered and patchily distributed in the marrow space. However, most lacunae (more than 50 %) are filled with viable osteocytes. Occasionally, some empty lacunae are seen at this stage probably due to a diminished blood supply, even though technical artifacts (suboptimal tissue fixation or decalcification) or aging phenomenon may result in the loss of staining of osteocytic nuclei [40].
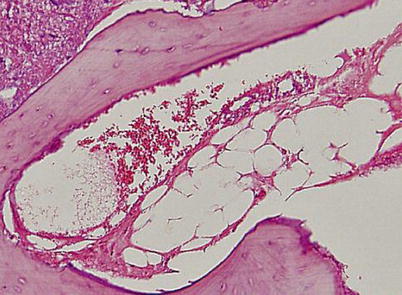

Fig. 18.2
Nuclear staining of marrow fat cells is not seen and the marrow space is necrotic. However, most lacunae (more than 50 %) are filled with viable osteocytes (Arlet and Durroux Type 2 lesion) (hematoxylin and eosin stain, original magnification ×100)
In this stage, sequestrum, a focal lesion of completely dead marrow cells and osteocytes, is not formed, and there is no evidence of reparative processes at the periphery of the necrotic zone. Thus, MRI shows no focal lesion in the femoral head. However, angiography shows an arterial interruption, and bone scan shows a cold lesion in the femoral head [26].
When the duration of ischemia is temporary and shorter than the threshold for complete osteocytic death, this lesion is not progressive and remains as so-called borderline necrosis [10].
However, when the ischemia is prolonged with a total lack of perfusion beyond the threshold of osteocytic death, the ischemic lesion progresses to ON.
18.4.3 Thrombophilia and Hypofibrinolysis
Various hereditary and genetic conditions, which cause increased thrombosis and/or impaired fibrinolysis, accentuate and prolong microvascular coagulation.
Protein C has an anticoagulant function by degrading procoagulant factors Va and VIIIa. Protein S serves as cofactor for activated protein C [41]. Protein C and protein S deficiencies [41–43] as well as mutations in the factor V Leiden or the prothrombin 20210A gene [44, 45] increase thrombosis. Polymorphisms of the plasminogen activator inhibitor-1 gene (PAI-1) are associated with hypercoagulable state [41, 46, 47].
18.4.4 Impaired Angiogenesis
The marrow necrosis triggers a reparative process including reactive angiogenesis. An impairment of angiogenesis, as well as thrombophilia/hypofibrinolysis, has been proposed as a mechanism for developing ON. Nitric oxide, which was originally discovered as a vasodilator product of the endothelium, promotes angiogenesis and bone formation. Polymorphism in the nitric oxide synthase gene is associated with idiopathic ON [15, 19]. Vascular endothelial growth factor (VEGF), which is induced by hypoxia, is a strong angiogenic protein and also plays a role in the formation of cartilage and bone. Genetic polymorphisms of VEGF are associated with the development of steroid-induced ON [17, 20]. Steroids also impair angiogenesis by suppressing the production of VEGF [54].
18.4.5 Osteocytic Death and Sequestrum Formation
Thrombophilia/hypofibrinolysis and impaired angiogenesis may lead to a prolonged anoxia and sequestrum formation in the femoral head.
After the onset of ischemia, osteocytes begin to disappear within 2–5 days and completely disappear within 2–4 weeks [39, 40, 55–58].
Once most osteocytes die and a sequestrum is formed, the lesion is irreversible and progresses to definite ON (Arlet and Durroux Type 3 lesion) (Fig. 18.3). However, if the necrotic region is small (<1 cm), it may heal spontaneously.
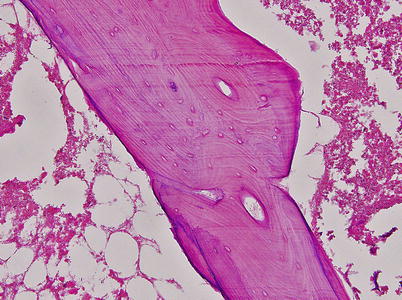

Fig. 18.3
The marrow space is necrotic and most lacunae are empty (Arlet and Durroux Type 3 lesion) (hematoxylin and eosin stain, original magnification ×100)
18.4.6 Reparative Process at the Margin of the Sequestrum
In a usual reparative process such as fracture healing, a fibrous vascular ingrowth occurs in the necrotic region. Vascular canals penetrate the medullary canals of the cancellous bone and the Haversian canals in the overlying cortical bone. Primitive mesenchymal cells infiltrate into the necrotic portion, which differentiate into osteoblasts and osteoclasts. Immature woven bone is deposited on the dead trabecular bone, which is slowly resorbed by the process of so-called creeping substitution [59].
However, creeping substitution does not occur after the formation of sequestrum. Instead, a foreign body reaction occurs at the peripheral portion of the sequestrum. Histiocytes and giant cells aggregate around the sequestrum, forming a fibrous capsule (Arlet and Durroux Type 4 lesion) (Fig. 18.4). This capsule is called a reactive zone, which appears as a band lesion on MRI. A definite diagnosis of ON can be made when there is a focal sequestrum surrounded by a peripheral reactive zone [10]. At this stage, the reactive band appears on MRI.
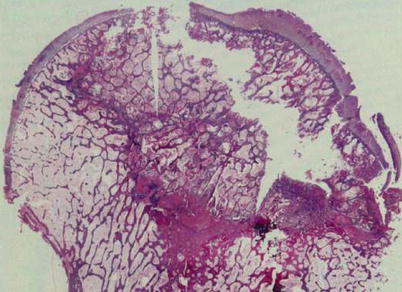

Fig. 18.4
A foreign body reaction is seen at the peripheral portion of the sequestrum. Histiocytes and giant cells aggregate forming a fibrous capsule (Arlet and Durroux Type 4 lesion) (hematoxylin and eosin stain, original magnification ×100)
Around this reactive zone, marrow edema often occurs. During the presence of edema, bone marrow pressure is elevated and patients have hip pain [60].
Although vessels penetrate into the fibrotic capsule, the angiogenesis is blocked at the margin of sequestrum [61], and reparative fibrovascular tissue cannot penetrate into the marrow space of the sequestrum. Thus, no biological repair process occurs in the necrotic portion. The fibrotic capsule is gradually substituted by newly formed bone by osteoblasts and osteocytes, which appears as a sclerotic rim on radiographs.
18.4.7 Saponification and Collapse of Necrotic Bone
Although there is no biological reaction in the necrotic portion, dead marrow undergoes a chemical change. Dead adipocytes release fatty acids, which saponify with extracellular calcium to form insoluble soaps [62–64]. Saponified fats and other necrotic areas eventually calcify, which appear as Mitchell class D lesion (dark signal on T1- and T2-weighted images) on MRI [65]. The dead trabeculae and saponified marrow do not attain the previous mechanical strength and structural integrity. Even with protected weight-bearing load, fatigue fracture occurs at the subchondral portion, which appears as a crescent sign on the radiograph, leading to collapse of the femoral head. Ultimately, this leads to a depression of articular cartilage and degenerative arthritis of the hip.
18.5 Borderline Necrosis
After an intravascular coagulation and marrow necrosis, a complete fibrinolysis and sufficient compensatory angiogenesis may occur within the critical ischemic period. In this situation, most osteocytes (>50 %) remain viable (Arlet and Durroux Type 2 or 3 lesion). The ischemic insult does not form a sequestrum and does not trigger a peripheral reparative process. This lesion is called “borderline necrosis” [10]. Borderline necrosis is seen in the femoral head of patients who have been exposed to risk factors without developing ON and in the contralateral femoral head of patients who have osteonecrosis in one hip.
In borderline necrosis, the reactive band, which is the earliest MR finding for ON, is not seen. The only nonspecific MR finding is an increase of signal intensity of the proximal femoral metaphysis in T1-weighted image, which means a conversion of hematopoietic to fatty marrow and increase of fat content. Angiography shows interruption of superior retinacular artery or medial femoral circumflex artery. Bone marrow pressure is slightly elevated, and bone scan shows decrease of radionuclide uptake. Although some patients have mild hip pain at the initial period of ischemia, most patients are asymptomatic.
The borderline necrosis does not progress to ON (Fig. 18.5).
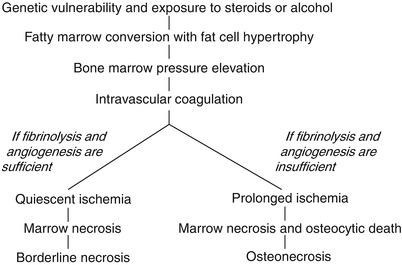

Fig. 18.5
Pathways of borderline necrosis and osteonecrosis
18.6 Bone Marrow Edema Syndrome
Bone marrow edema syndrome (BMES) is a rare disease of the hip, which regresses spontaneously within several months [23, 66–71].
Although the exact etiology is unknown, ischemia seems to be the most probable cause because histologic findings are similar to those of early-stage ON [23, 67, 68].
It frequently affects pregnant women in their third trimester [66, 69], and an association with hypofibrinolysis was reported in familial BMES [70, 71]. However, most patients with BMES do not have risk factors for ON [23, 58–68].
Intravascular coagulation and marrow necrosis may occur in the absence or paucity of risk factors. In this situation, the subsequent events after the ischemia are quite different from ON. Complete fibrinolysis and reactive vasodilatation occur [72]. Thus, there is only a brisk period of subthreshold ischemic hypoxia. Reactive hyperemia and increased permeability lead to an interstitial edema in the marrow space.
Although the marrow is necrotic, most osteocytes remain viable, and most lacunae are filled with osteocyte, because the ischemia is not so severe to induce complete bone death. There is no sequestrum formation and no reactive zone around the necrotic portion [23, 68].
A creeping substitution occurs in the regions of marrow necrosis. Fibrovascular tissue and dilated vessels are seen in the medullary cavity. Immature woven bone is deposited on the surface of trabecular bone. The marrow space is filled with fluid. By the Arlet and Durroux classification, these lesions are Type 2 or Type 3 lesions (Fig. 18.6).
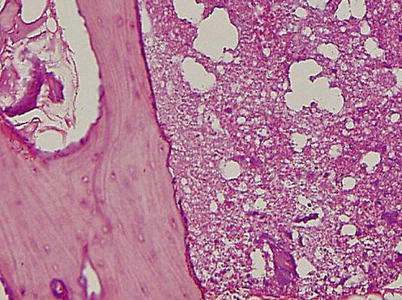

Fig. 18.6
The marrow space is filled with fluid. Fibrovascular tissue and dilated vessels are seen in the marrow space. Immature woven bone is deposited on the surface of trabecular bone. Although the marrow is necrotic, most osteocytes remain viable and most lacunae are filled with osteocytes (hematoxylin and eosin stain, original magnification ×100)
During the edema, bone marrow pressure is elevated. Most patients suffer hip pain and are diagnosed at this stage. MRI shows findings of edema in the marrow space. Angiography shows arterial dilatation, and bone scan shows increased uptake in the proximal femur.
The increased perfusion induces transient demineralization of the trabeculae and cortical bone of the proximal femur. When the demineralization is severe, a nonspecific radiolucency of the proximal femur appears on the radiograph. However, the bone trabeculae have normal volume density and no signs of “osteoporosis.” Because of this, Wilson et al. proposed to use the term “transient BMES” instead of “transient osteoporosis” [73].
As the intraosseous vascularity and perfusion return to normal, the osteoid mineralizes and the lesion is spontaneously healed.
Bone marrow edema syndrome is a self-limiting disease and does not progress to ON.
References
1.
Arlet J. Nontraumatic avascular necrosis of the femoral head. Past, present, and future. Clin Orthop Relat Res. 1992;277:12–21.PubMed
2.
Mont MA, Hungerford DS. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am. 1995;77(3):459–74.PubMed
3.
4.
Prosnitz LR, Lawson JP, Friedlaender GE, Farber LR, Pezzimenti JF. Avascular necrosis of bone in Hodgkin’s disease patients treated with combined modality therapy. Cancer. 1981;47(12):2793–7.PubMedPubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree