Pathophysiology of Traumatic Brain Injury and Impact on Management
4.1 Introduction
The damage wrought by traumatic brain injury (TBI) is a dynamic process that occurs during many stages, only some of which can be mitigated by medical or surgical intervention. Injury begins at the time of trauma (or even before if the risk factors for injury, such as intoxication, are considered) and continues as the body reacts to the initial injury. This statement applies not only to damage resulting from the TBI itself but also to the sequelae of systemic injury, such as hypotension resulting from hemodynamic shock. This chapter focuses on the mechanism of tissue damage after TBI, including both the primary effects of trauma on the brain and the secondary injuries that can result from the body’s response to injury. Although preventative measures, such as wearing protective headgear, can lessen the severity of the primary injury, once the injury has occurred, there is little medical treatment can do to alter or reverse the effects.
Medical or surgical treatment does play a role in diminishing secondary injury. Secondary injury is damage caused by the body’s response to the primary insult; that is, the primary injury may be a skull fracture with accumulation of an epidural hematoma. The secondary injury results from mass effect of the hemorrhage, with compression on important structures. Secondary injury also can result from inflammatory mediators that alter metabolism.
Systemic factors also contribute to secondary injury after TBI. These factors include hypoxia, hypothermia, hypotension, and hypercoagulable state.1 The Brain Trauma Foundation has specific guidelines for addressing the management of these factors,2 some of which are directly applicable to neuroimaging.
This chapter discusses the mechanism of primary injury to alert radiologists to important imaging findings that may suggest associated injuries or lead to secondary injury. Additionally, this chapter details imaging findings of secondary brain injury, including edema, infarction, and brain herniation. The concept of cerebral autoregulation is introduced. Finally, physiologic monitoring devices used in the intensive care unit for the care of these patients are discussed, with imaging correlates.
4.2 Primary Brain Injury
The effect of primary injury to the brain depends on the nature of the trauma, including the mechanism of injury and the force and direction of impact. Systemic factors, including hypoxia, shock, coagulopathy, and effects of drugs or alcohol, can impact how the primary injury develops.
Injury resulting from a direct blow to the head results from mechanical forces on the skull, underlying vascular structures, and brain parenchyma. Mechanical force to the skull may cause deformation of the calvarium, sometimes resulting in fracture. Compression or tearing of blood vessel walls underlying the force of impact can result in hemorrhage.3 The location of the injured vessels will result in the specific pattern of brain injury, including extra-axial or intra-axial hemorrhage. The underlying brain parenchyma may sustain a contusion or laceration. Additional structures underlying the impact zone may also be damaged.
Brain contusions occur on the surface of the brain and result from injury to small vessels and neural tissue (▶ Fig. 4.1). Contusions may be produced by mechanical compression of tissue beneath an area of skull depression caused by mechanical force or by sudden negative pressure when the calvarium snaps back into place.3 Factors such as whether the head is moving at the time of impact and whether the head is supported (such as against the ground) at impact affect the pattern of contusions.
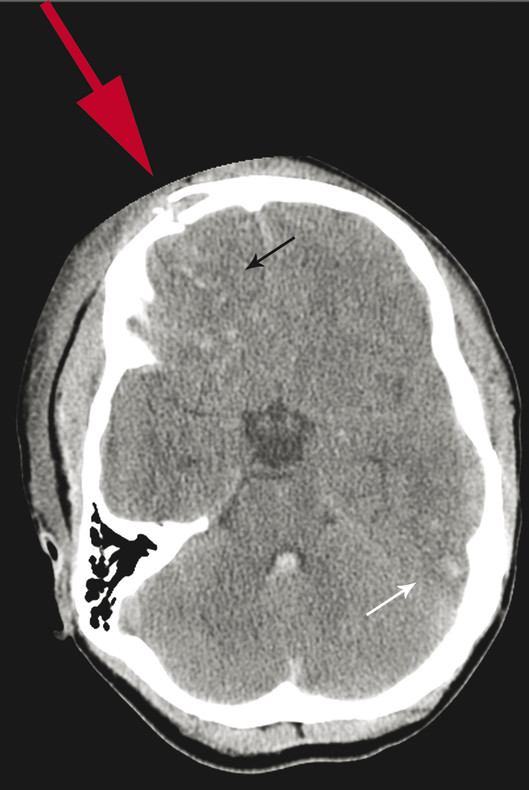
Fig. 4.1 Computed tomography showing sequelae of right frontal impact (red arrow) with fractures of the inner and outer table of the frontal sinus, underlying parenchymal contusions (black arrow), and contrecoup contusions (white arrow).
Although the calvarium provides a good primary defense against blunt trauma to the head, in some cases, the calvarium may also be a means of damage to the brain, such as occurs in the case of contrecoup injury. With sudden deceleration (such as when the skull hits the floor), the inertia of the brain results in a secondary impact of the brain against the opposite inner table of the skull, which can result in hemorrhage of the underlying brain parenchyma and contrecoup contusions (▶ Fig. 4.1). Gliding contusions result when the brain strikes the rigid falx cerebri.
Contusions occur as a result of bleeding within the brain parenchyma. The term laceration may be used if the overlying pial membrane is disrupted. Initially, damaged blood vessels result in bleeding into the tissue. Local mass effect or vessel thrombosis can then result in ischemic necrosis.3 Inflammation results as the body responds to the injured tissue, inciting a leukocytic and lymphocytic response and also an inflammatory cytokine response. Damaged tissue may undergo apoptosis or necrosis.4 With time, the body removes the damaged tissue, leaving reactive gliosis. The imaging appearance and characteristic locations of brain contusions are discussed further in Chapter ▶ 3, Neuroimaging of Traumatic Brain Injury.
At a cellular level, the mechanism of brain parenchymal injury is due to excitotoxicity, mediated via excessive release of excitatory neurotransmitters, including glutamate.4,5 Extracellular glutamate binds N-methyl-d-aspartate and AMPA receptors and allows sodium and calcium influxes into the cell. Calcium influx then results in activation of calcium-dependent enzymes, resulting in further cell damage.4 Mitochondrial dysfunction also plays a role.6
Damage to extra-axial blood vessels also results in TBI. Laceration to the meningeal arteries can result in an epidural hematoma, and laceration to the middle meningeal artery in particular can result in rapid accumulation of epidural hematoma with associated mass effect on the underlying brain. Less commonly, epidural hematomas result from injury to the venous sinuses.
Subdural hematomas are caused by laceration of bridging veins from the brain surface to the dura. As a result of direct bleeding, subdural hematomas also can occur in conjunction with adjacent cerebral contusions or lacerations7,8 or as a result of laceration of a cortical artery or branch.8–10 Unlike patients with epidural hematomas, those with subdural hematomas commonly sustain associated injuries to the underlying brain parenchyma, which may explain why they experience less favorable outcomes despite early decompressive surgery.7
Critical structures within the cranial vault may be damaged by a direct impact, for example, the cochlea (▶ Fig. 4.2a,b). In some cases, the primary injury may spare the brain, but the injury may put the brain at risk for further injury. For example, as discussed in Chapter ▶ 10, Maxillofacial Trauma, a frontal sinus fracture extending through an inner table may increase the risk for subsequent brain infection. An arterial injury may result in a rapidly expanding hematoma, compressing surrounding brain structures, or in downstream infarction (▶ Fig. 4.2c–e). Injury to the transverse sinus may result in epidural hematoma (▶ Fig. 4.2f,g) and risk subsequent venous sinus thrombosis and venous infarction. Thus, it is important to consider not only what structures are damaged but also how that injury may put the patient at risk for subsequent complications.
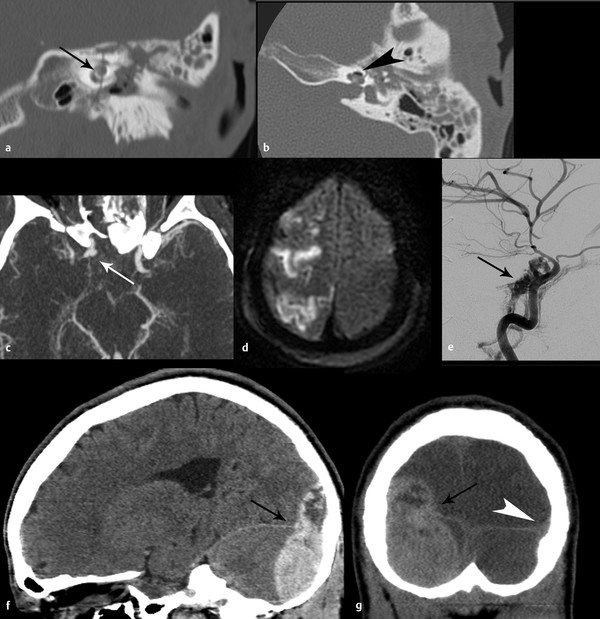
Fig. 4.2 Direct mechanical trauma in three patients. Coronal (a) and axial (b) computed tomography (CT) of the left temporal bone showing fracture line traversing the cochlea (black arrow) with pneumolabyrinth (black arrowhead) axial CT angiogram (c) shows right internal carotid artery pseudoaneurysm (white arrow) related to severe displaced skull-base fracture (not shown), resulting in infarcts [diffusion-weighted imaging magnetic resonance imaging (MRI)] (d). High-flow carotid-cavernous fistula developed within 1 week (e) (black arrow). Sagittal (f) and coronal (g) CT shows posterior fossa epidural hematoma (EDH) resulting from surgically proven sigmoid and transverse sinus laceration. Overlying skull fracture is not shown. Note the relationship of the EDH (black arrow) to the expected location of the transverse sinus (normal contralateral sinus marked with white arrowhead). (g).
In some cases, there is a rotational component of force applied to the brain. Shear, tensile, and compressive strains on the brain tissue may result in axonal injury. This torsional component can disrupt the fine structures of the brain, resulting in diffuse axonal injury.3 Damage to axons is a complex process that depends on the type and duration of mechanical trauma and may occur by different mechanisms.11 The imaging manifestations of diffuse axonal injury may be minor, especially as seen by CT, with the extent of injury evident only by histologic review. The imaging findings of diffuse axonal injury are discussed in Chapter ▶ 3, Neuroimaging of Traumatic Brain Injury.
4.3 Secondary Brain Injury
Secondary brain injury occurs after the primary insult as a result of the physiologic response of the brain to injury. The primary culprits behind secondary brain injury are mass effect, brain swelling, and ischemia.
Brain swelling after TBI occurs as a result of cellular edema and is associated with elevated intracranial pressure (ICP).12 The onset of cerebral edema is variable and may occur late. Certain populations, for example, children, appear more susceptible to severe cerebral edema after TBI.13 If severe, cerebral edema can result in brain herniation and put the patient at risk for the attendant complications (discussed later in this chapter). CT findings of cerebral edema include small ventricles and subarachnoid spaces, including compression or effaced perimesencephalic (basilar) cisterns (▶ Fig. 4.3).13
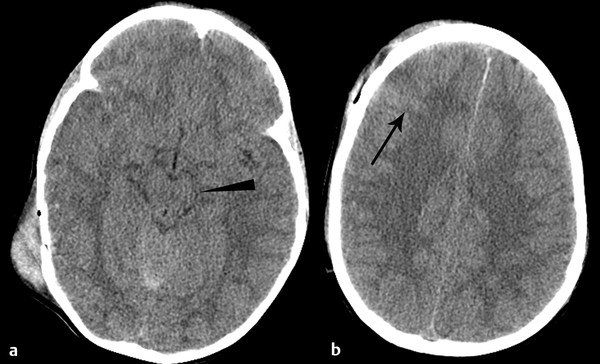
Fig. 4.3 Cerebral edema. Axial computed tomography in a 4-year-old child shows diffuse effacement of the sulci and basilar cisterns (a) (arrowhead), consistent with cerebral edema. Note the traumatic convexal subarachnoid hemorrhage (b) (arrow).
Brain herniation occurs when mass effect from either a focal mass lesion, such as a hematoma, or generalized increased brain volume, as in the case of cerebral edema, pushes the brain against and around the dural structures that are in place normally to hold the brain in position. Depending on the location of the additional mass, the pattern of herniation will vary.
4.3.1 Subfalcine Herniation
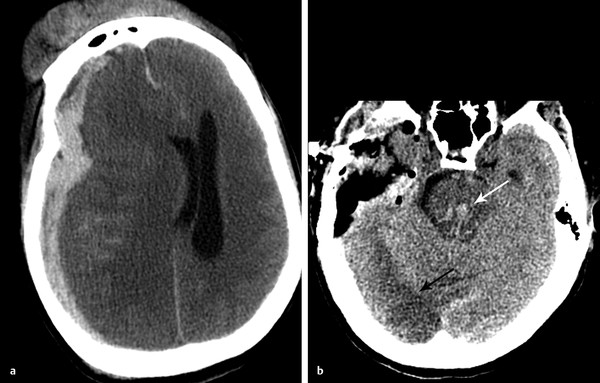
Subfalcine herniation occurs with mass effect from a frontal or parietal lesion and is commonly associated with acute subdural hematoma. Subfalcine herniation occurs when the cingulate gyrus is pushed beneath the rigid falx cerebri (▶ Fig. 4.4). Subfalcine herniation is best measured by assessing for midline shift on the axial image. A line should be drawn from the anterior to the posterior leaves of the falx. The distance between this line and the septum pellucidum is a reliable measure of midline shift. Although the falx is a rigid structure, the falx will deflect, given enough pressure (▶ Fig. 4.4). When severe, or when the location of mass is more inferior, midline shift may be best measured at the level of the third ventricle.
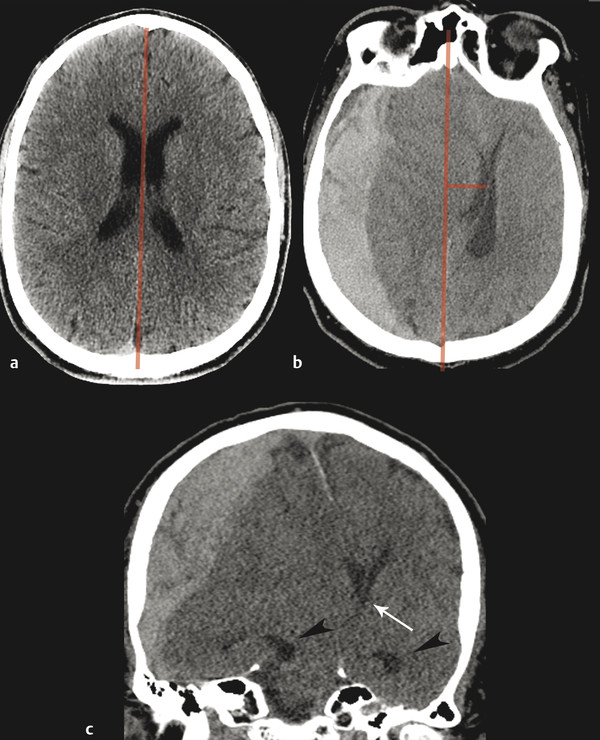
Fig. 4.4 Subfalcine herniation resulting from acute subdural hematoma. Normal computed tomographic imaging (CT) (a) demonstrates midline septum pellucidum, with line drawn from the anterior to the posterior attachment of the falx cerebri. Subfalcine herniation on axial CT (b), showing midline shift (red lines). Coronal reformat (c) demonstrates leftward herniation under the deflected falx cerebri. Note the mass effect on the foramina of Monro (white arrow) and dilation of the temporal horns of the lateral ventricles (arrowheads), out of proportion to sulci, indicating obstructive hydrocephalus.
Subfalcine herniation is common and may be well tolerated by patients in whom mass effect has developed over time, such as in the case of brain tumor. When acute, subfalcine herniation can have significant complications and require emergent surgical decompression. Signs of significant herniation include entrapment of the lateral ventricles. This usually first affects the contralateral side and occurs when there is compression of the foramen of Monro, thereby blocking egress of cerebrospinal fluid (CSF) into the third ventricle. There is subsequent enlargement of the entrapped lateral ventricle, which may be best seen by evaluating the size of the temporal horns and comparing to the size of the sulci (▶ Fig. 4.4).
Vascular complications of subfalcine herniation also occur when there is compression of the ipsilateral anterior cerebral artery between the herniating brain and the falx cerebri. If not relieved, this type of herniation may result in infarct (▶ Fig. 4.5). Even without a frank large vessel territory infarct, the presence of midline shift has been associated with a decreased cerebral metabolic rate of oxygen.14
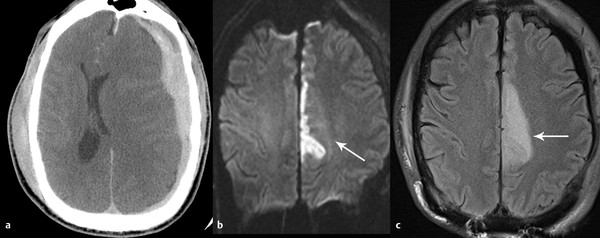
Fig. 4.5 Anterior cerebral artery infarct that is due to subfalcine herniation. Computed tomography (CT) at presentation (a) demonstrates acute left subdural hematoma with significant subfalcine herniation. The patient was immediately taken to surgery and decompressed but developed left anterior cerebral artery infarct evident on diffusion-weighted imaging (b) and fluid-attenuated inversion recovery (FLAIR) (c) sequence.
4.3.2 Downward Tentorial Herniation
Tentorial herniation occurs when there is downward pressure on the brain against the tentorium cerebelli, ultimately resulting in downward displacement of the brain through the tentorial incisura. If the mass effect is predominantly temporal, the first structure to herniate will be the uncus, a medially directed bulge of tissue along the mesial temporal lobe (▶ Fig. 4.6).
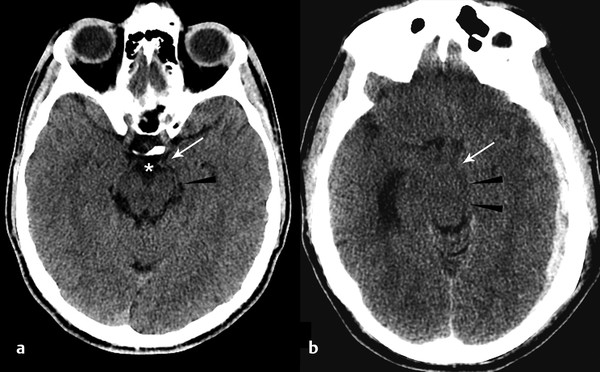
Fig. 4.6 Uncal herniation resulting from acute subdural hemorrhage. Normal computed tomography (CT) (a) demonstrates the normal configuration of the uncus (arrow) and ambient cistern (arrowhead). Note that the suprasellar cistern is patent (*). Uncal herniation on CT (b) manifests as medial displacement of the uncus (arrow), effacement of the ambient cisterns (arrowhead), and suprasellar cisterns. Note enlargement of the contralateral temporal horn of the lateral ventricle, consistent with entrapment.
To assess for uncal herniation, evaluate the position of the uncus with respect to the suprasellar cistern. Whereas medial placement of the uncus alone may indicate impending uncal herniation, when severe, the uncus is clearly medially deviated and effaces the lateral margin of the suprasellar cistern (▶ Fig. 4.6). Clinically, uncal herniation may be marked by dilatation of the ipsilateral pupil (“blown pupil”), caused by compression of the ipsilateral third nerve against the free edge of the tentorium.
Central transtentorial herniation occurs as the result of more central or more severe mass effect (▶ Fig. 4.7); it may follow subfalcine herniation.15 For central transtentorial herniation, one or both parahippocampal gyri may slip inferiorly past the tentorial margin. To assess for central transtentorial herniation, evaluate the ambient cistern, the CSF space surrounding the midbrain. Young patients in general will have more brain parenchyma and smaller CSF spaces than older adults, but even in children there should be some CSF evident surrounding the brainstem. When mild, cisterns may only be compressed (i.e. narrowed), but with progressive herniation, basilar cisterns become completed effaced (i.e., absent) (▶ Fig. 4.7).
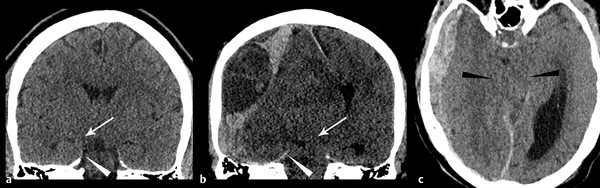
Fig. 4.7 Transtentorial herniation due to acute subdural hematoma (SDH) with hyperacute components. Normal coronal computed tomography (a) demonstrates normal position of the parahippocampal gyrus above tentorium cerebelli (arrowhead). With large acute and hyperacute SDH (b), there is medial and downward shifting of the right parahippocampal gyrus (white arrow) over the free edge of the tentorium (white arrowhead). Axial image (c) demonstrates complete effacement of the basilar cisterns with narrowing of the midbrain in the transverse dimension (black arrowheads).
Significant tentorial herniation can cause compression of the adjacent midbrain, which may be deviated away from the side of herniation.16 The contralateral cerebral peduncle will be compressed against the opposite side of the tentorium. If severe, transtentorial herniation will cause compression of the midbrain in the transverse dimension.16
With sufficient mass effect, the entire brainstem may be pushed downward, causing traction on the structures and fiber tracts of the brainstem. Traction on the perforating arteries arising from the basilar artery may result in tearing of these vessels with development of Duret hemorrhages in the midbrain and upper pons (▶ Fig. 4.8).17 This type of hemorrhage is a sign of grave prognosis.