CHAPTER 5 Patterns of Contrast Enhancement
Contrast material has been essential to cross-sectional neuroimaging for almost 4 decades. The first intravascular contrast agents were U.S. Food and Drug Administration (FDA)–approved urographic and angiographic iodine-based compounds for parenteral injection. Modern iodine-based agents for CT are now usually low- and iso-osmolar compounds designed to lower the frequency of side effects and provide a higher safety margin. Multiple gadolinium-based contrast agents have been developed, and six have been approved by the FDA for intravascular injection for contrast-enhanced MRI: Vasovist Injection; Magnevist; MultiHance; Omniscan; OptiMARK; and ProHance.
In the central nervous system (CNS) contrast enhancement is produced by two related, yet independent, processes: interstitial (extravascular) enhancement and vascular (intravascular) enhancement.1,2 The brain, spinal cord, and nerves are supplied by capillaries that have a selectively permeable membrane that creates a “blood-brain barrier.” This selective barrier protects the nervous system from certain plasma proteins and limits inflammation by blocking inflammatory cells from entering the tissue. The primary structure of the blood-brain barrier is from endothelial cell specialization produced by cooperation between these cells and the astrocyte foot processes. The normal blood-brain barrier includes a continuous basement membrane, narrow intercellular gaps with junctional complexes, and only rare pinocytosis. The normal intact blood-brain barrier is far more permeable to lipophilic compounds (as measured by octanol/water partition fraction), and the blood-brain barrier retards lipophobic compounds. Some “desirable” compounds, such as glucose, are facilitated to cross the vessel wall or are actively transported out of the vessel and into the tissue compartment. Vascular enhancement is a combined product of blood volume, blood flow (delivery of contrast agent or “wash-in”), and “mean transit time” or time needed for “washout” of a contrast agent. In addition to neovascularity, which increases both blood volume and blood flow, vasodilatation of existing normal vessels (hyperemia) produces increased intravascular enhancement.
MRI after administration of a contrast agent has several important differences, as compared with CT enhancement. Many MR pulse sequences create a “flow void phenomena”; thus, high-flow lesions such as aneurysms and vascular shunts (e.g., arteriovenous malformations) will have very low signal intensity.3 The aneurysmal dilation of the vein of Galen, dural and pial fistulas, and the more common arteriovenous malformations will show as spherical, tubular, or serpentine signal voids.
Many different physiologic and pathologic conditions demonstrate contrast enhancement. Angiogenesis and neovascularity in neoplastic masses, breakdown of the blood-brain barrier in both infectious and noninfectious inflammation, physiologic changes from cerebral ischemia, and capillary pressure overload (eclampsia and hypertension) will all affect the blood-brain barrier and increase permeability. A paralysis of autoregulation—the primary cause of “malignant brain edema”—is actually a reactive hyperemia and will enhance on MRI, CT, and even at angiography. The newly created vessels from tumor angiogenesis will increase both blood volume and blood flow as compared with contralateral normal brain tissue. There will also be a short mean transit time.
EXTRA-AXIAL ENHANCEMENT
Extra-axial enhancement in the CNS may be classified as either pachymeningeal or leptomeningeal. The pachymeninges (from the Greek for “thick” meninges) are the dura mater, which comprises two fused membranes derived from the embryonic meninx primativa: the periosteum of the inner table of the skull and a meningeal layer. Pachymeningeal enhancement may be manifested up against the bone, or it may involve the dural reflections of the falx cerebri, tentorium cerebelli, falx cerebelli, and cavernous sinus. The leptomeninges (Greek for “skinny” meninges) are the pia and arachnoid. Leptomeningeal enhancement may occur on the surface of the brain or in the subarachnoid space. Because the normal, thin arachnoid membrane is attached to the inner surface of the dura mater, the pachymeningeal pattern of enhancement is also described as dura-arachnoid enhancement (Fig. 5-1). In comparison, enhancement on the surface of the brain is called pial or pia-arachnoid enhancement. The enhancement follows along the pial surface of the brain and fills the subarachnoid spaces of the sulci and cisterns. This pattern is often referred to as leptomeningeal enhancement and is usually described as having a “gyriform” or “serpentine” appearance.
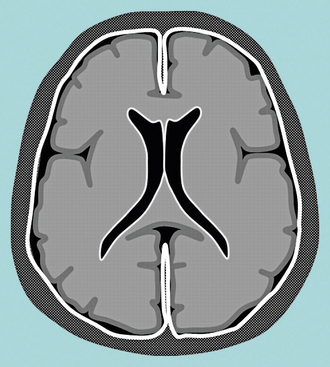
FIGURE 5-1 Schematic diagram of dura-arachnoid enhancement on MRI. This is also called pachymeningeal enhancement.
(From Smirniotopoulos JG, Murphy FM, Rushing EJ, et al. Patterns of contrast enhancement in the brain and meninges. RadioGraphics 2007; 27:525-551.)
Pachymeningeal or Dura-Arachnoid Enhancement
Because the vessels supplying the dura do not have a blood-brain barrier, both endogenous and exogenous compounds (e.g., albumin, hemosiderin, contrast agents) readily leak into the interstitial space. Enhancement of the dura is normal on CT, typically uniform in the falx and tentorium. Enhancement of the dura against the skull is not readily noticed because of the adjacent dense white line of the cortical bone of the inner table of the skull. In contrast, on contrast-enhanced T1W MR images, the normal dura shows only thin, linear, and discontinuous enhancement.4
A variety of processes may accentuate dural enhancement, including vasocongestion and intradural edema, both of which may be nonspecific reactions to a wide variety of benign or malignant processes, including transient postoperative changes, intracranial hypotension (Fig. 5-2), neoplasms such as meningiomas, metastatic disease (from breast and prostate cancer), secondary CNS lymphoma, and granulomatous disease. Because of the Monro-Kellie doctrine,5 when the cerebrospinal fluid pressure drops, there may be secondary fluid shifts that increase the volume of capacitance veins in the subarachnoid space. After neurosurgical intervention, even the placement of a shunt catheter or intracranial pressure bolt, meningeal enhancement is very frequent and occurs in a majority of patients. The postoperative enhancement may be pachymeningeal (dura-arachnoid) or leptomeningeal (pia-arachnoid)6 and can be localized to the side of the procedure or diffuse (bilateral, supratentorial and infratentorial). Extra-axial enhancement may also occur after uncomplicated lumbar puncture in about 5% of patients.7 Patients with spontaneous intracranial hypotension, with or without a cerebrospinal fluid leak, may show generalized diffuse pachymeningeal enhancement.4,8,9 Prolonged intracranial hypotension may lead to vasocongestion and interstitial edema in the dura mater, findings similar to those seen in the dural tail of a meningioma. Rarely, a skull fracture may cause a cerebrospinal fluid leak and intracranial hypotension. More often intracranial hypotension may be related to a (seemingly) uncomplicated lumbar puncture. However, in most cases no definitive cause is ever found, and it is described as “idiopathic” intracranial hypotension.
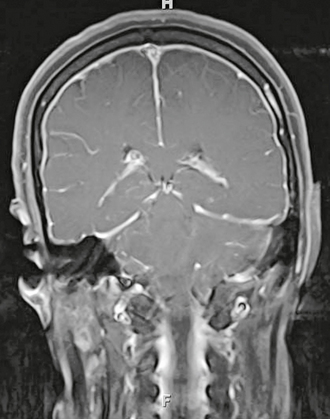
FIGURE 5-2 Coronal T1W MR gadolinium-enhanced image of idiopathic intracranial hypotension. There is diffuse, smooth, and linear dura-arachnoid enhancement. Although veins may also enhance normally, there should be no other subarachnoid enhancement.
MRI is relatively sensitive and specific in the detection of benign or spontaneous intracranial hypotension. A typical clinical feature of spontaneous intracranial hypotension is a headache that is orthostatic (postural) and worse when the patient is upright. Imaging findings include thickened dura with linear enhancement of the pachymeninges both above and below the tentorium, no enhancement of the sulci or brain surface, enlargement of the pituitary gland, and descent of the brain (low cerebellar tonsils and downward displacement of the iter of the third ventricle below the tentorial incisura line).9 Some patients may have additional features of subdural effusions or even subdural hemorrhage. Other features of intracranial hypotension include dural thickening and an enlarged pituitary gland. Leptomeningeal enhancement (within the sulci) may be seen postoperatively but is not common with spontaneous intracranial hypotension and could suggest leptomeningitis, either inflammatory or neoplastic.
Extra-axial neoplasms may produce pachymeningeal enhancement. The most common primary dural neoplasm is meningioma, a benign tumor of meningothelial cells (Figs. 5-3 and 5-4). Meningiomas are slow-growing, well-localized, World Health Organization (WHO) grade 1 lesions that are usually resectable for cure.10–12 They typically manifest in patients in the fourth to sixth decades of life, and they are roughly twice as common in women as in men. The typical meningioma is a localized lesion with a broad base of dural attachment (see Fig. 5-5B). This neoplasm actually arises from the arachnoid membrane that is attached to the inner layer of the dura mater. Even in the early days of CT, the accuracy of cross-sectional imaging in the detection and characterization of meningioma was very good.13 Contrast-enhanced MRI demonstrates a new finding (one not observed at CT): the dural tail or “dural flare.” The dural tail is a curvilinear region of dural enhancement adjacent to the bulky hemispheric tumor (Figs. 5-5 to 5-7; see also Fig. 5-4).14–16 The finding was originally thought to represent dural infiltration by tumor, and resection of all enhancing dura mater was thought to be appropriate.17 Several studies have confirmed that in most cases of meningioma, linear dural enhancement is most likely a reactive process18 rather than neoplastic, especially when it was more than a centimeter away from the bulky part of the tumor. The dural reaction may include a combination of increased extravascular spaces as well as small vessel vasocongestion. Both will thicken the dura, and the increased interstitial water allows visualization of contrast enhancement (see Fig. 5-5) because even normal dural capillaries do not form a blood-brain barrier. In addition to primary dural neoplasms, such as meningioma, hemangiopericytoma, and solitary fibrous tumor, metastases are possible. In women, breast carcinoma can cause a solitary dural metastasis; and in men, prostate cancer can do the same. Secondary CNS lymphoma is usually extra-axial and may be dural based or fill the subarachnoid space. Granulomatous inflammatory and infectious diseases including sarcoid, tuberculosis, Wegener’s granulomatosus, luetic gummas, rheumatoid nodules, and fungal disease produce pachymeningeal enhancement usually involving the basilar meninges, including the suprasellar cistern and vessels of the circle of Willis. Sarcoid may produce focal or diffuse dural thickening (see Fig. 5-3).
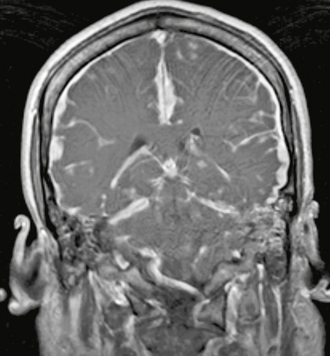
FIGURE 5-3 Coronal T1W MR gadolinium-enhanced image of dural sarcoid. There is grossly abnormal diffuse thickening and enhancement of the dura including both the falx and the tentorium.
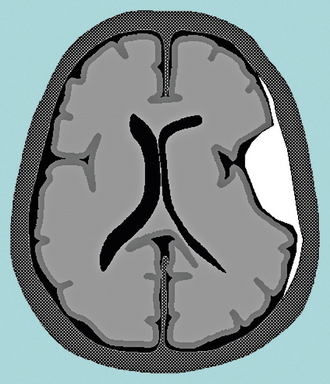
FIGURE 5-4 Schematic diagram of dural-based enhancement limited to a mass growing against the inner table of the skull. This is a typical pattern for a meningioma, with linear tapering enhancement from the “dural tail.”
(From Smirniotopoulos JG, Murphy FM, Rushing EJ, et al. Patterns of contrast enhancement in the brain and meninges. RadioGraphics 2007; 27:525-551.)
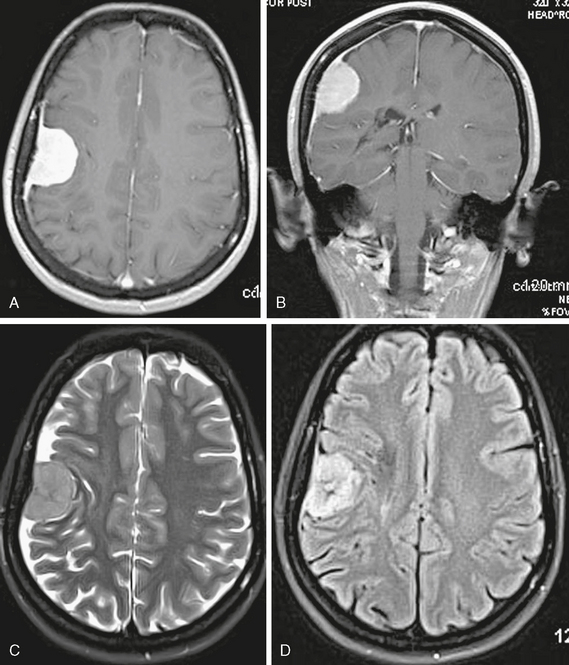
FIGURE 5-5 Axial (A) and coronal (B) T1W MR gadolinium-enhanced images of a meningioma. There is a brightly enhancing dural-based mass with a hemispheric shape. A long area of tapering linear enhancement—”dural tail”—extends away from the central bulky mass. Most of this linear enhancement is reactive rather than neoplastic. Axial T2W (C) and FLAIR (D) images show clearly that the lesion is extra-axial.
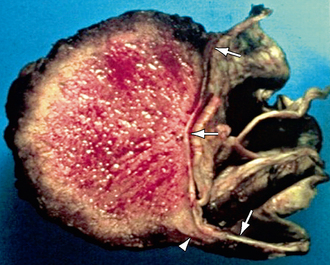
FIGURE 5-6 Meningioma. The specimen has been cut in half, showing a hemispheric mass affixed to the underlying dura (arrows). There is a “claw” of tumor growing along the dura (arrowhead). However, the enhancement seen on MRI was far more extensive.
(From Smirniotopoulos JG, Murphy FM, Rushing EJ, et al. Patterns of contrast enhancement in the brain and meninges. RadioGraphics 2007; 27:525-551.)
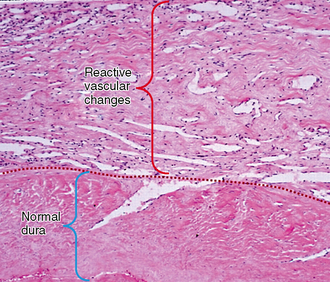
FIGURE 5-7 Meningioma. Dural tail (H&E, original magnification, ×250). The lower half shows normal dura mater—mostly collagen. Vascular reactive changes and venous congestion, along with interstitial edema, contribute to contrast enhancement.
(From Smirniotopoulos JG, Murphy FM, Rushing EJ, et al. Patterns of contrast enhancement in the brain and meninges. RadioGraphics 2007; 27:525-551.)
Leptomeningeal or Pia-Arachnoid Enhancement
When the abnormal enhancement extends into the subarachnoid spaces of the sulci and cisterns it is called leptomeningeal or “pia-arachnoid” enhancement. Bacterial, viral, and even fungal meningitides may cause leptomeningeal enhancement (Fig. 5-8). The primary mechanism for this enhancement is a breakdown of the blood-brain barrier of the pial vessels themselves. In bacterial meningitis, glycoproteins released by bacteria cause breakdown of the blood-brain barrier and allow contrast material to leak from vessels into the cerebrospinal fluid. Bacterial and viral meningitis exhibit enhancement that is typically thin and linear (Figs. 5-9 and 5-10).19 Some cases of fungal meningitis may produce nodular or “lumpy” enhancement.
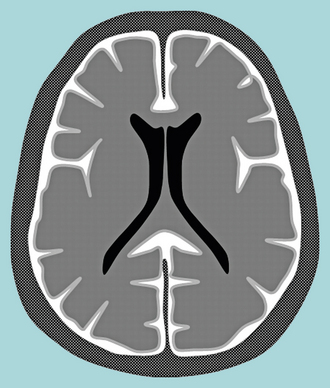
FIGURE 5-8 Schematic of pia-arachnoid (subarachnoid) enhancement. The contrast material fills the subarachnoid space and enters the sulci between the cerebral and cerebellar gyri. This pattern occurs in both bacterial meningitis and cerebrospinal fluid dissemination of neoplasms—”carcinomatous” meningitis.
(From Smirniotopoulos JG, Murphy FM, Rushing EJ, et al. Patterns of contrast enhancement in the brain and meninges. RadioGraphics 2007; 27:525-551.)
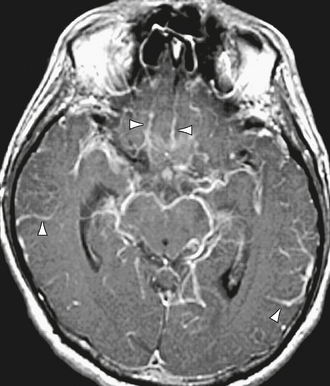
FIGURE 5-9 Axial T1W MR gadolinium-enhanced image of bacterial meningitis. There is diffuse linear superficial (pial) enhancement in the subarachnoid space, extending into sulci (arrowheads) and along the surface of the midbrain.
(From Smirniotopoulos JG, Murphy FM, Rushing EJ, et al. Patterns of contrast enhancement in the brain and meninges. RadioGraphics 2007; 27:525-551.)
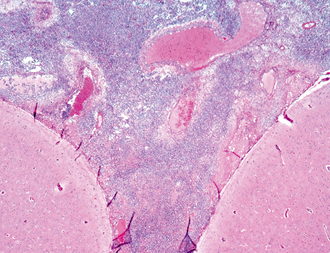
FIGURE 5-10 Streptococcus pneumoniae meningitis (H&E, original magnification, ×400). There is a dense inflammatory infiltrate along the surface of the brain that fills the subarachnoid space.
(From Smirniotopoulos JG, Murphy FM, Rushing EJ, et al. Patterns of contrast enhancement in the brain and meninges. RadioGraphics 2007; 27:525-551.)
Neoplasms that disseminate by spreading into the subarachnoid space—“carcinomatous meningitis”—also produce enhancement of the brain surface and subarachnoid space (Fig. 5-11). Primary brain tumors that reach the ventricular or pial surface may spread this way, including medulloblastoma, ependymoma, choroid plexus papilloma/carcinoma, glioblastoma, germinoma, and oligodendroglioma, as well as secondary tumors (e.g., lymphoma and breast cancer. We expect neoplastic disease in the subarachnoid space to produce thicker, lumpy, or nodular enhancement, similar to that of fungal disease. However, despite this logic, neoplastic meningitis can appear surprisingly thin and linear.
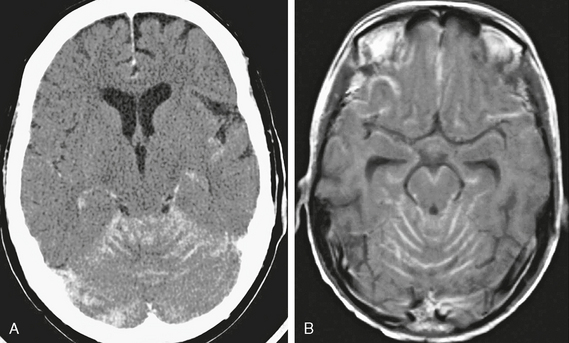
FIGURE 5-11 Carcinomatous meningitis. This patient has diffuse subarachnoid spread of medulloblastoma. Axial contrast-enhanced CT scan (A) and axial T1W MR gadolinium-enhanced image (B) both show abnormal yet linear enhancement in the sulci of the cerebellum, forming a coating around the brain stem.
(From Smirniotopoulos JG, Murphy FM, Rushing EJ, et al. Patterns of contrast enhancement in the brain and meninges. RadioGraphics 2007; 27:525-551.)
INTRA-AXIAL ENHANCEMENT
Gyral Enhancement
Gyral enhancement—along the surface of the brain—is almost always vascular or inflammatory and only rarely neoplastic (Fig. 5-12). Vascular causes of brain parenchymal gyral enhancement include causes of vasoactive hyperemia: migraine, loss of autoregulation, posterior-reversible encephalopathy syndrome, and reperfusion after thrombolysis or spontaneous clot lysis/migration. Gyral enhancement due to blood-brain barrier breakdown is also seen with reperfusion, during the subacute “healing” phase after cerebral infarction, and with vasculitis and encephalitis. Enhancement from hyperperfusion can be seen after seizures. The differential diagnosis between vascular and inflammatory causes of a superficial and serpentine pattern of enhancement requires clinical correlation and analysis of the enhancement topography. Embolic events and most thrombotic strokes have an abrupt onset of symptoms and involve regions that map to vascular territories. Migraine headache has a characteristic aura and throbbing pain. Fever, indolent history, and nonspecific headache or lethargy may suggest an encephalitis. Gyral lesions affecting the territory of a single artery are often vascular, whereas inflammatory lesions may affect multiple territories. The most common vascular processes affect the middle cerebral artery territory (up to 60% of cases). The lesions of posterior-reversible encephalopathy syndrome usually localize in the posterior cerebral artery territory, perhaps due to a deficiency in the sympathetic innervations (vaso nervorum).
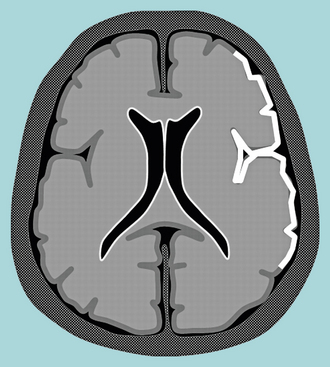
FIGURE 5-12 Schematic of gyral gray matter enhancement. The cortical gray matter enhances while the underlying white matter does not.
(From Smirniotopoulos JG, Murphy FM, Rushing EJ, et al. Patterns of contrast enhancement in the brain and meninges. RadioGraphics 2007; 27:525-551.)




