Pediatric Cancers
Chris Beltran
Thomas Merchant
Pediatric radiation therapy encompasses a wide variety of patient ages, diseases, treatment sites, and tumor volumes. The head-to-toe nature of treatment in this group of patients presents a broad array of immobilization and treatment verification challenges. Image-guided radiation therapy for pediatric patients is further distinguished by the use of anesthesia for a significant proportion of patients, the priority that is given to minimizing dose to normal tissues, and the low doses that are used for certain tumors, emphasizing the need to minimize uncertainty in target localization. The following four sites are relevant to the pediatric patient population:
Central nervous system
Retroperitoneum
Pelvis
Extremities
Pediatric radiation therapy is complicated by the extremes of age and body size. Pediatric treatment protocols now include planned irradiation of very young children, as young as 12 months of age, as well as young adults. Patient weight may vary from 10 to 100 kg. Immobilization and localization options are fewer in very young or uncooperative patients because they must be anesthetized for planning and therapy to ensure precise irradiation of target volumes that have been designed to achieve minimal irradiation of normal tissues. Minimizing dose to normal tissue and limiting the side effects of irradiation are major considerations in treatment planning and delivery regardless of patient age, sites of disease, and other factors coincident with the planned treatment regimen. Ionizing radiation has wide-ranging side effects in pediatric patients involving cognition,1,2 bone growth,3 and malignancy induction.4 When considering localization and verification, all nontherapeutic irradiation must be justified and minimized.
This chapter is divided into four parts. The first part consists of general background information and introduces basic terminology. The second part is devoted to targeting and the different imaging modalities that are used to aid in contouring target volumes and critical structures. The third part describes the use of serial on-treatment imaging to evaluate change in the shape and volume of patient and tumor. The final part describes the methods used to localize pediatric patients during treatment and their limitations. The chapter concludes with a brief summary.
BACKGROUND
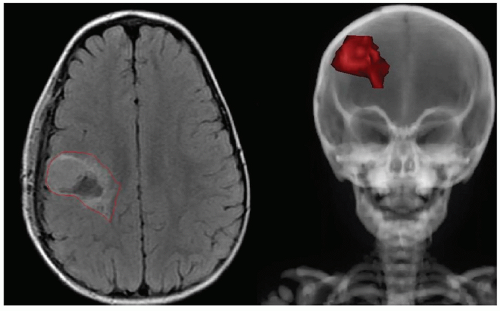
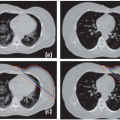
Nearly one third of the 12,000 children diagnosed with cancer each year in the United States will receive external-beam radiation therapy (EBRT) as part of their initial management. In modern pediatric protocols, the volume targeted to receive the prescription dose, the planning target volume (PTV), is defined based on the specific diagnosis, location and extent of disease, relevant imaging studies, and clinical and treatment-related factors including prior surgery, the use of chemotherapy, and the risk of treatment-related side effects. Considering International Commission on Radiation Units and Measurements (ICRU)-50 and ICRU-62 definitions,5,6 relevant imaging studies are required to define the gross tumor volume (GTV), which is expanded by an anatomically confined margin to form the clinical target volume (CTV). The CTV is meant to account for potential subclinical invasion of the tumor and defines the volume at risk. Ideally, the CTV would receive a tumoricidal dose of radiation, and no other tissue would be irradiated.
Unfortunately, this ideal situation cannot be achieved because the tumor is often imbedded within or adjacent to organs at risk (OARs). Irradiated OARs may limit the dose that can be prescribed without serious complications. In addition, due to possible temporal variation in the position, shape, and/or size of the CTV, an internal margin (IM) must be taken into account, ideally on serially acquired on-treatment imaging. A setup margin (SM) is also required to account for all the variations and uncertainties in the daily patient positioning and beam delivery. Minimization of the SM may be achieved using various daily localization schemes. The IM and SM are combined and added to the CTV, giving rise to the PTV. An IM and SM are also added to each OAR to give a planning risk volume (PRV).
The need to understand these margins has become increasingly clear as high-dose conformal radiation therapy, including intensity-modulated radiation therapy (IMRT)7 and proton therapy,8 enter the mainstream for children. Given the importance and complexity of determining proper margins, numerous citations propose population-based formulas for margin calculations9, 10, 11, 12, 13, 14, 15, 16 for the SM portion of the PTV.
The SMs required for various adult sites and localization techniques have also received considerable attention. The sites that have been studied include head and neck,17, 18, 19, 20 brain,21 liver,22,23 prostate,24, 25, 26, 27, 28 pelvis,29 and lung.30, 31, 32 Different modalities of
target localizations have been researched, including ultrasound,24,33, 34, 35 electronic portal imaging,21,25,26,36, 37, 38 stereoscopic kilovoltage (kV) imaging,39 implanted radiofrequency transponders,40,41 in-treatment room computed tomography (CT),42,43 and cone beam CT (CBCT).44,45 The effects of breathing22,23,46 and intrafraction motion22,47 have also been assessed in the adult population.
target localizations have been researched, including ultrasound,24,33, 34, 35 electronic portal imaging,21,25,26,36, 37, 38 stereoscopic kilovoltage (kV) imaging,39 implanted radiofrequency transponders,40,41 in-treatment room computed tomography (CT),42,43 and cone beam CT (CBCT).44,45 The effects of breathing22,23,46 and intrafraction motion22,47 have also been assessed in the adult population.