Hyaline Membrane Disease (Aka Surfactant Deficiency Disease)
Congenital Bowel Abnormalities
Conditions in Older Children with Cystic Fibrosis
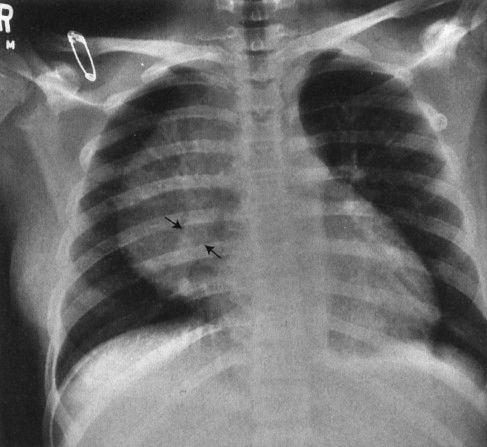
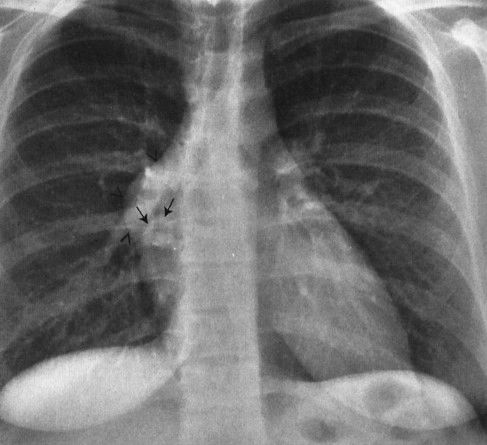
Children are not merely small adults. Sure, the body parts (hearts, eyes, noses) are the same, but the fact that children are growing and changing subjects them to different diseases as well as to different structural appearances. Chest radiographs of young children, for instance, feature that ubiquitous but often misdiagnosed anterior mediastinal mass, the thymus (Fig. 4.1). This organ, important in the immune response, usually becomes inconspicuous by age 5 or so; however, its involution is extremely variable, and it is not uncommon to find thymic remnants on chest computed tomography (CT) scans up to age 20 years (Figs. 4.2 and 4.3). The thymus is a living piece of tissue that changes its configuration in a number of ways. In response to stress, it may shrink. When indented by the ribs, it may form a wavy border; in pathologic conditions, such as a pneumomediastinum, it may even be displaced superiorly and laterally over the lung fields (Figs. 4.4 to 4.6). The first rule in looking at children’s radiographs is to expect change and variation and consider those factors before inventing a disorder that isn’t real.
In general, congenital abnormalities are much more likely to present as clinical problems in neonates than they are in adults. Another way to look at it is if you got to adulthood without a congenital anomaly bothering you, it is likely that you will carry that anomaly to your grave. When you deal with an abnormal abdomen in a neonate, a congenital anomaly is extremely likely; in a 4-year-old it is somewhat likely; and in a 15-year-old it is less likely. If you play by the 99% rule in an 80-year-old, you probably shouldn’t even think of congenital abnormalities as the cause of an acute abdomen. Having said this, I know that everyone will be able to find the unusual case of a congenital defect causing grief to an 80-year-old, but remember that’s the zebra, not the horse!
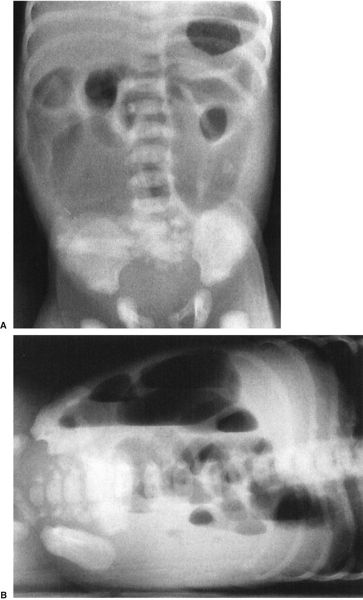
Abdominal radiographs of neonates are very different from those of adults, whereas radiographs of older children and teenagers begin to have a lot of similarities with those of adults. Abdominal films of neonates are especially discrepant because of a number of physiologic factors. First and foremost, neonates swallow a tremendous amount of air during their relatively inefficient breathing and eating. It is, therefore, not at all unusual to find many loops of small bowel in the plain film of a normal neonate (Fig. 4.7), whereas in an adult or older child it is unusual to see so much small bowel gas (Fig. 4.8). In fact, it is abnormal to see a gasless abdomen in a neonate! Such a finding usually means that the infant is so obtunded that it cannot swallow air, that there is a discontinuity of the gastrointestinal (GI) tract preventing air from entering the bowel, or that the infant is septic or otherwise critically ill (Fig. 4.9). All of this air in the small bowel makes the interpretation of the films difficult as far as determining bowel distention. The best rule to remember is that the bowel loops of a normal neonate are thin walled and lie in close proximity to each other. The appearance of thick-walled bowel or marked separation of the bowel loops suggests an abnormal intra-abdominal process (Fig. 4.10). Comparison of Figs. 4.7 and 4.10 illustrates this point.
The haustra of the colon are notoriously variable in their development and do not become prominent until about 6 months of age. For this reason, trying to differentiate large from small bowel on the plain radiographs of a neonate’s abdomen is fraught with difficulty. Occasionally, you can get lucky and be reasonably certain in differentiation; however, most of the time it isn’t even worth guessing. This makes the determination of whether there is rectal gas or not even more critical. The vast majority of newborns will have gas all the way through their GI tract by 24 hours after birth. If there is any doubt as to whether a child has rectal gas or has obstruction, the prone cross-table lateral film is invaluable in making this distinction (Fig. 4.11). Remember: if you are looking for distal gas in an infant in whom you are considering bowel obstruction, go right for the rectum! (Table 4.1).
Another big difference between neonates and older children or adults is the presence of the belly button. This necessary structure and the appurtenances appended to it make for some weird shadows on abdominal films of babies. Many an unsuspecting physician has called an umbilical clamp a bone or a foreign body (Fig. 4.12). The umbilicus itself protrudes much farther in a neonate than in an adult. Any coin-shaped lesion in the lower midabdomen of a neonate should be considered the umbilical remnant until proven otherwise. A good clue is that, owing to the air surrounding the protruding umbilical stump, the edges of the umbilicus are very sharply defined, particularly the inferior edge (Fig. 4.13).
FIGURE 4.1 The large superior mediastinal mass that bulges both to the right and to the left is the thymus in this normal 3-month-old infant.
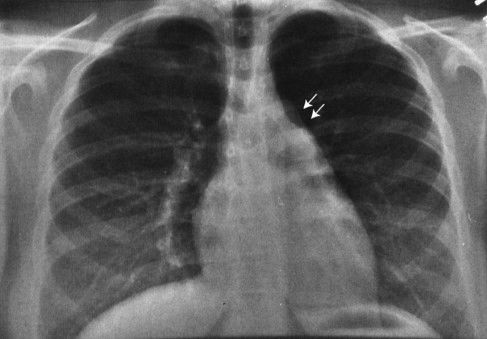
FIGURE 4.2 This teenager has a clearly visible thymic edge (arrows) on chest radiograph. Visualization of the thymus in normal teenagers is an unusual, but not rare, finding.

FIGURE 4.3 Chest CT of a 16-year-old patient shows a large thymus anterior to the opacified vessels. Note how the left lobe of the thymus (T) sticks out to abut the lung (arrows).
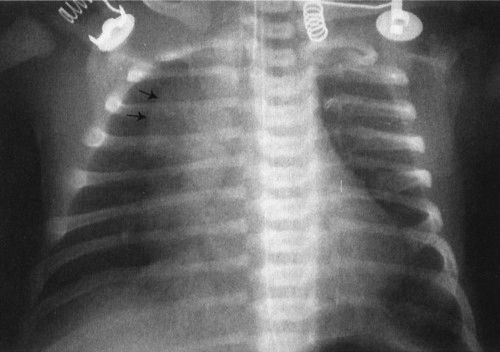
FIGURE 4.4 The large anterior mediastinal mass with the irregular margin (arrows) is the thymus. The thymic wave sign or undulating thymic border occurs because the costal cartilages are made of more firm tissue than the thymus; therefore, the thymus itself is indented. This is a normal finding.
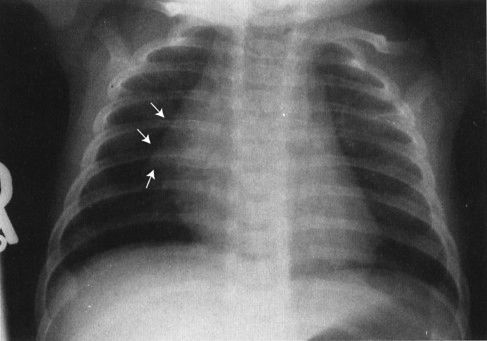
FIGURE 4.5 The so-called thymic sail sign shows the sharp edge of the thymus, somewhat like a boat sail, projected against the lung.
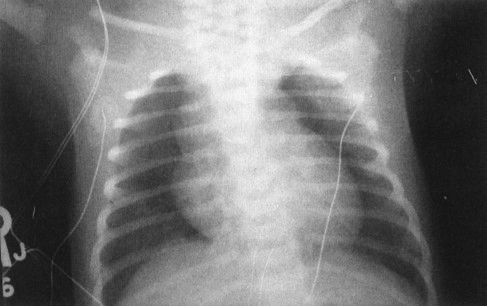
FIGURE 4.6 The large superior mediastinal mass in this otherwise well-term neonate is the thymus. If you call anything in the anterior superior mediastinum of a neonate normal thymus, you will be right 99% of the time.
FIGURE 4.7 This abdominal film shows the bowel gas pattern of a normal newborn baby. Notice that there are a number of nondistended bowel loops with considerable small bowel gas. The loops lie next to each other and are thin walled. This is a good visual picture to remember for the normal bowel gas appearance of a neonate. Older children do not have this much gas, and adults have very little small bowel gas, visible on plain films. Contrast this with Fig. 4.9, a baby with sepsis and some bowel wall edema.
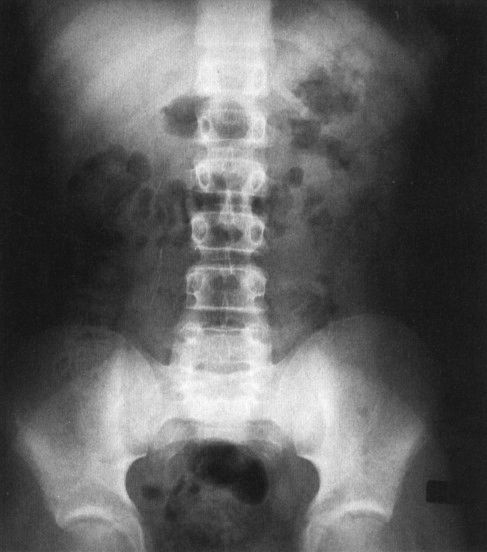
FIGURE 4.8 This plain film of the abdomen was obtained on a normal child with constipation. There is gas in the colon and stomach but very little gas in the small bowel. Contrast this to the appearance of Fig. 4.7, the neonate, where there is normally considerable small bowel gas.

FIGURE 4.9 Gasless abdomen in a very ill baby. There is almost always some gas in the stomach but very little gas distal. This is abnormal and can occur as a result of the baby being too ill to swallow or a systemic illness. In this case, the baby was immobilized so that he would not fight the ventilator and this gasless abdomen resulted. Normally, babies should have gas in both large and small bowels.
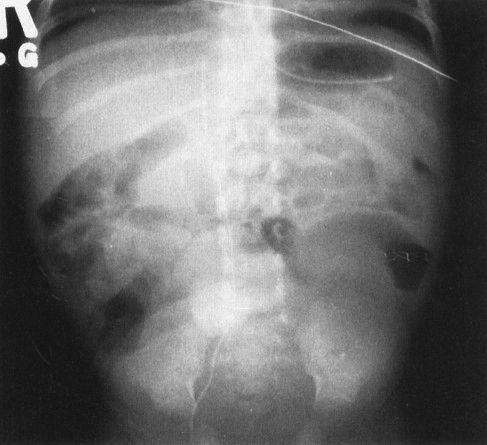
FIGURE 4.10 Contrast the abdominal plain film in this critically ill and septic neonate with the normal bubbly pattern of bowel gas shown in Fig. 4.7. The loops of bowel are separated, and some of the bowel shows evidence of a moderate degree of dilation. These findings are nonspecific and can be seen in any severely ill infant. In this case, sepsis caused abnormal bowel motility in this abnormal appearing radiograph.
FIGURE 4.11 A prone cross-lateral view of the abdomen can often be helpful in showing whether or not gas is present in the rectum. Remember that because you cannot tell large bowel from small bowel in neonates, it is important to identify the rectum when you are trying to look for distal gas. Rectal gas is usually identified in the hollow of the sacrum, as in this infant.
In this chapter I will discuss some of the common radiographic diagnoses of children, first neonates, then the older child. The intent is not to be comprehensive, as there are 1000-page tomes for that; rather, this is intended to show practical imaging approaches to common diagnoses.
The newborn’s chest radiograph is a complex study with a substantial number of differences from that of an adult (Fig. 4.14). All babies have to change from an intrauterine environment where their lungs are fluid filled to one where they are breathing air. This transformation, which must occur within moments of birth, involves interaction of the pulmonary lymphatics, capillary vessels, and chest compression. This normal biologic process is not always smooth. In fact, many babies, if not all, have some very short-lived tachypnea in the first minute or two after being born, owing to the vagaries of clearing their normal in utero lung fluid. The physiologic phenomenon is reflected as the pleural effusions and streaky densities seen in the lungs on radiographs taken shortly after birth.
CAUSES OF BOWEL OBSTRUCTION IN THE NEONATE
Atresia |
Malrotation |
Hernia |
Meconium ileus |
Hirschsprung’s disease |
Bowel duplications |
In an insignificant number of babies, it takes longer than a few moments to clear all of the in utero lung fluid. This condition has been aptly termed transient tachypnea of the newborn (TTN; Fig. 4.15). TTN should resolve clinically and radiographically within 24 hours, leaving behind a normal chest. In the first hours of life, however, this picture leads to a great clinical quandary because the radiograph of the neonate with TTN is indistinguishable from the radiograph of early neonatal pneumonia. There are a few clues, such as the presence of a large pleural effusion, which favors pneumonia; however, a definitive distinction can never be made. Here is a classic health care conundrum. TTN is much more common than neonatal pneumonia; however, conventional diagnostic tests cannot separate the two. The outcome of an untreated neonatal pneumonia is dire, so what do you do? Most prudent physicians bite the bullet and treat, knowing that in many instances the antibiotics are unnecessary. This situation demonstrates the principle that if the perceived severity of possible outcome is great, it alters the treatment choice when diagnosis is ambiguous. In other words, if you cannot tell, but the patient might die if you don’t treat, overtreating is acceptable.
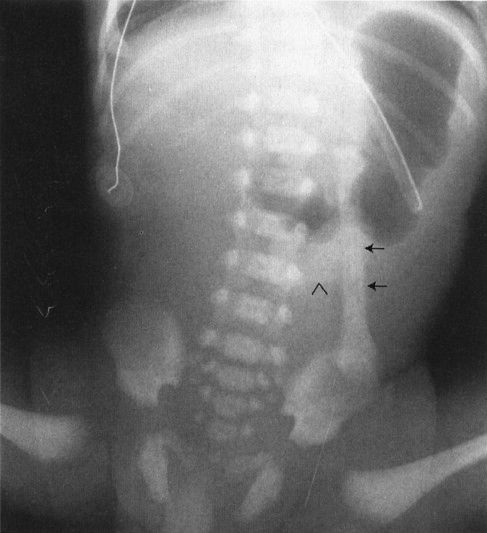
FIGURE 4.12 A newborn infant who has just begun to swallow air. Notice the nasogastric tube marking the stomach. The oblong structure to the left of the spine (arrows) almost looks like a bone of some sort; however, it is clearly attached to the umbilical stump (arrowhead) and in fact represents an umbilical cord clamp superimposed on the abdominal film.
FIGURE 4.13 This baby undergoing an upper GI has a circular, bowel-filled mass in the lower midabdomen. The mass projects slightly to the left because the baby is in an oblique position (arrows). Note the very sharp margin, indicating that the mass protrudes off the abdominal wall. Anytime you see an extremely sharp border on a plain film of the abdomen, there has to be either air or fat surrounding that structure. In this case, air surrounds the structure because the umbilicus protrudes out from the abdominal wall. This is a typical umbilical hernia.
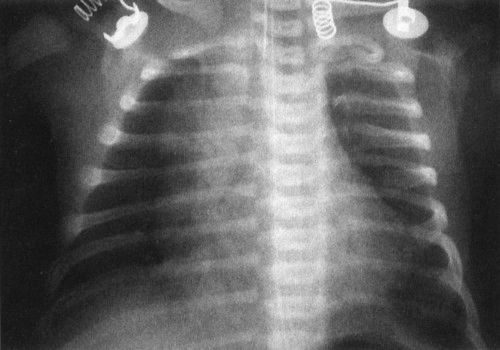
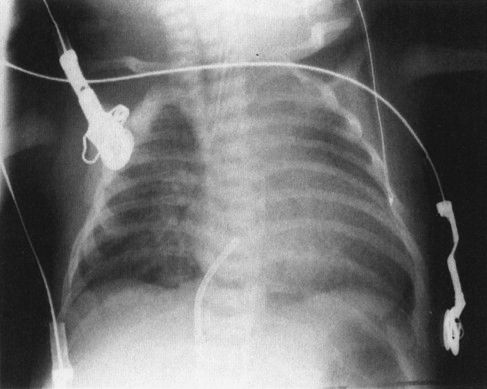
FIGURE 4.14 A normal newborn infant’s chest. Notice the very different appearance from an adult chest. First, the heart and thymus are much larger than the cardiomediastinal silhouette of an adult. If you measure the heart and thymus (cardiothymic structures) and compare them to the diameter of the chest, often a baby’s cardiothymic ratio will exceed 60% of the chest diameter. This is still normal. Notice also that the bones are very different, with a number of growth plates and other variants owing to infant development.
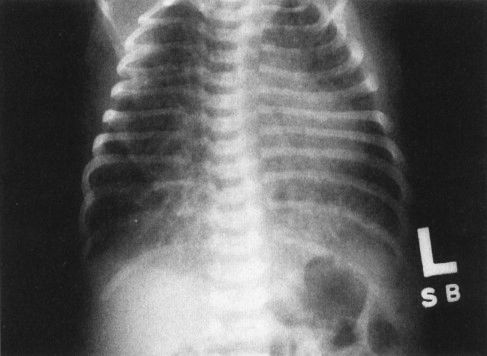
FIGURE 4.15 Chest film of a 4-hour-old baby shows bilateral streaky densities whose distribution is asymmetric. Note that the lungs are also very hyperinflated. All this cleared within 24 hours, and the findings were due to transient tachypnea of the newborn. On this film alone, however, you cannot exclude pneumonia. It is necessary to have the follow-up film to confirm the diagnosis of transient tachypnea.
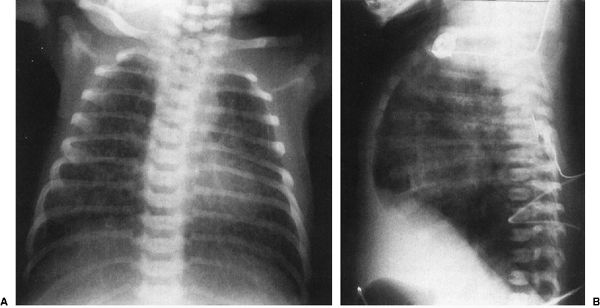
There are other neonatal lung diseases that exhibit radiographs showing bilateral streaky densities. TTN is the prototype for this appearance; however, neonatal pneumonia, neonatal venous stasis, and neonatal congestive heart failure can be mimicked. A few points of differentiation are possible. A large pleural effusion usually favors pneumonia and specifically reactive pneumonia, owing to group B streptococcal infection (Fig. 4.16). Most neonatal pneumonias are acquired during the birth process so that these pneumonias start small and tend to get dramatically worse over the first few days of life. If the streaky densities are associated with marked overaeration of the lungs, one might think of meconium aspiration. This disorder occurs when the newborns release their sphincters, spilling meconium into the amniotic fluid. This sphincter release, a response to stress, usually occurs in utero and the meconium is aspirated as the child begins to try to breathe. This is a particularly nasty pneumonia because the meconium is both irritating and viscous so that it obstructs the airways as well as causing the reactive pneumonia. One of the tips for diagnosing meconium aspiration is the massive hyperinflation of the lungs usually seen on the chest film (Fig. 4.17).
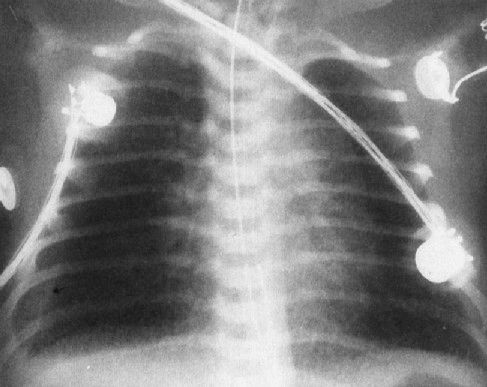
FIGURE 4.16 A very ill newborn with the streaky density pattern in both lungs and a large pleural effusion (arrow) on the right. Pneumonia plus a pleural effusion usually means group B streptococcal infection in neonates.
FIGURE 4.17 The anterior–posterior radiograph (A) and lateral radiograph (B) of a 1-day-old infant with severe meconium aspiration, showing marked hyperinflation of the lungs and bilateral streaky densities in both lung fields consistent with meconium aspiration. Notice on the lateral view (B) how flat the diaphragms are and how prominent the anterior–posterior diameter of the chest is. Meconium aspiration is highly associated with air-block phenomenon.
Babies can get congestive heart failure for a number of reasons, some of which involve intrinsic heart disease and many of which do not (Fig. 4.18). Arrhythmias, anemia, and arteriovenous shunts can cause high-output failure that is indistinguishable by chest film from the failure caused by intrinsic heart lesions such as hypoplastic left heart. The best clue that the streaky density pattern you are looking at on the chest film owing to heart disease is cardiomegaly. Beyond that, it is difficult to be more precise. The most important thing is to remember heart failure in your differential diagnosis.
HYALINE MEMBRANE DISEASE (AKA SURFACTANT DEFICIENCY DISEASE)
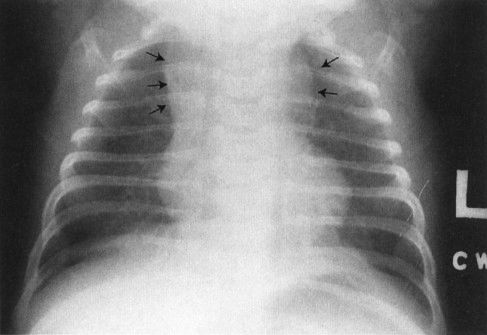
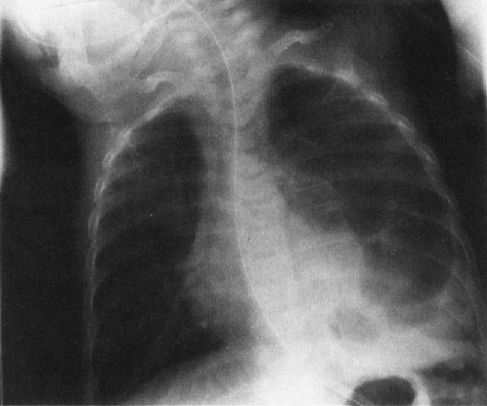
A common disorder of neonates is hyaline membrane disease (HMD). Here the clinical information helps a lot, as most of these infants are premature and most do not have respiratory distress immediately after birth. The other good news is that the radiographs are virtually diagnostic in the vast majority of cases (Fig. 4.19). The HMD radiograph shows four characteristic features: (a) diffuse granularity, (b) uniform disease, (c) air bronchograms, and (d) a relatively small lung volume. Not all radiographs will show all of these features, but most will have at least three.
HMD occurs owing to a lack of the lipid chemical surfactant that is synthesized by the alveolar lining cells of term infants. These type 2 alveolar lining cells develop and mature during the third trimester of pregnancy; therefore, they are deficient among premature infants. Surfactant works by lowering the surface tension of the alveoli, allowing them to remain expanded. A simple analogy is that of a child’s bubble pipe. You have to add soap to the water in the pipe to change the surface tension or you cannot blow many bubbles! If the surfactant is not present in the neonate’s lung, the bubbles (alveoli) collapse. There you have it: The radiology of HMD is predominantly the radiology of profound atelectasis on an alveolar rather than a segmental level. In fact, some of the more forward-thinking clinicians propose changing the name of this disease to surfactant deficiency disorder.
Pediatric surgical disease of the chest of a neonate can be roughly defined as anything that needs prompt intervention (Table 4.2). By this definition, for example, a tension pneumothorax needing treatment with a chest tube is surgical disease. In assessing the newborn child’s chest radiograph when surgical disease is suspected, you must take two steps. First, identify which side is the more abnormal (most surgical conditions are unilateral). Second, determine the direction of shift of the mediastinum. This is best done by looking at the trachea, but the position of the heart and thymus can also be secondary clues (Fig. 4.20). As a general (99%) rule, surgical conditions will displace the mediastinum away from the more abnormal side. For example, in the instance of a diaphragmatic hernia (a condition owing to an in utero defect that allows the abdominal contents to protrude into the chest), the heart and mediastinum are clearly shifted away from the side of the hernia by the mass of the protruding guts (Fig. 4.21). Therefore, if you look at the radiograph and decide that the side with the bowel in the chest is the abnormal side, and then look at the mediastinal position, you can readily deduce that this is a surgical condition and an emergency.
FIGURE 4.18 A: Cardiomegaly with pulmonary congestion in a child with congestive heart failure. The findings are nonspecific, and failure can occur due to multiple causes. B: A lateral arteriogram of the head of the baby shown in A shows the carotid artery branches (arrows) connecting to a large venous sinus, creating an arterial venous fistula. This condition is called a vein of Galen aneurysm. The baby is in high-output congestive heart failure.
COMMON SURGICAL CHEST CONDITIONS IN NEONATES
Pneumothorax |
Diaphragmatic hernia |
Lobar emphysema |
Cystic adenomatoid malformation |
Pleural effusions (large) |
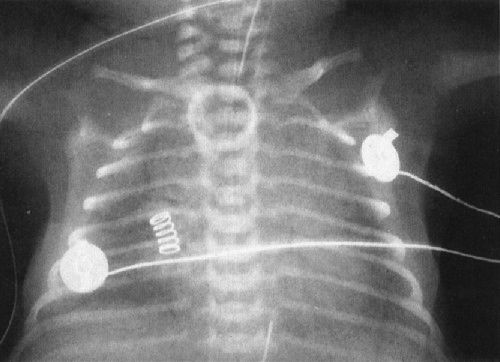
FIGURE 4.19 Classic radiograph of a patient with hyaline membrane disease. Note that the lungs are relatively small in volume.
FIGURE 4.20 A very ill neonate. Note that the bubbly mass in the left lung base causes the heart and mediastinum to be shifted from left to right. First you decide which lung is abnormal (in this case, clearly the left). If the heart and mediastinum are shifted away from the abnormal lung, it is almost always surgical disease of the chest. Incidentally, the mass in this case is a cystic adenomatoid malformation, a benign tumor of the lung caused by abnormal budding of the foregut.
When you are deciding on the more abnormal lung in neonates, remember that birth is a time of transition. In our example of a diaphragmatic hernia, in utero the bowel is filled with fluid. It isn’t until the infant begins to breathe and swallow air that the gut assumes its normal postnatal air-filled condition. Therefore, the first film in a neonate may show a fluid density filling the chest, but within a few moments the normal swallowing of air results in replacement of this fluid density by the bubbly appearance of air-filled bowel.
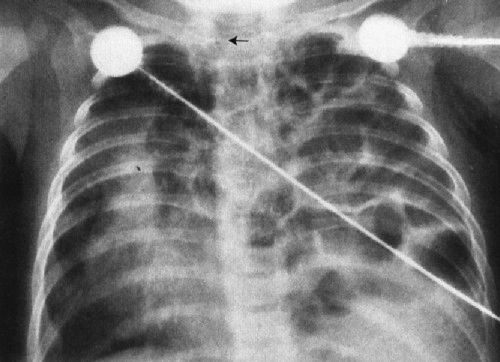
FIGURE 4.21 Bubbly material fills the left chest, displacing the heart and mediastinum far to the right. These bubbles actually are air-filled bowel. Notice the trachea area. Position of the trachea is the single best indicator of mediastinal displacement.
In some conditions this transition to an air-filled mass can be even further delayed until 2 or 3 days after birth. This usually occurs in situations in which the connection between the lung and the tracheobronchial tree is abnormal, and it can take a considerable period of time, up to several days, for the normal in utero lung fluid to empty out and the air to fill the lung anomaly. A good example of this is lobar emphysema, a condition whereby the tracheobronchial airway connects abnormally to a lobe of the lung, allowing air to flow in but not out. The lobe, therefore, hyperinflates, becoming a tumor in the chest. Babies don’t breathe air in utero; their lungs are fluid filled. Right after birth, this abnormally connected lobe is full of lung fluid as is the rest of the lung. The same mechanics that do not allow air to escape freely also do not allow the fluid to escape freely; therefore, it takes a long time for turnover of the fluid to take place, and the initial chest film looks like a solid (water) density mass with mediastinal displacement (Fig. 4.22). In a sense, it doesn’t make any difference because criteria one and two for diagnosing a surgical condition are met no matter what the status of the mass; however, it’s always nice to make a precise diagnosis.

FIGURE 4.22 A: A neonatal chest film shows mediastinal shift from left to right and a partially opaque fluid density in the left upper lobe. This is a patient with congenital lobar emphysema and only partial emptying of the fluid from the emphysematous lobe. B: The same patient at age 11 months demonstrates findings more typical of lobar emphysema with the markedly hyperinflated upper lobe herniated across the midline (arrows). The principle for surgical disease remains valid; mediastinal shift away from the abnormal side needs rapid intervention.
Our discussion so far has focused on lung disease, but of course there are other significant organs in the pediatric chest, including the heart and the esophagus. Esophageal abnormalities that are of importance in children are usually related to esophageal atresia. The most common form of esophageal atresia is a blind-ending proximal esophagus with a fistula extending from the trachea or left main stem bronchus to a blind distal esophagus. Inhaled air travels through the fistula and into the rest of the GI tract; therefore, the initial films can look superficially normal. The clues are that the GI tract is more distended by air than usual and the proximal esophageal pouch is very dilated. Clinicians become alerted to this condition when the child chokes on feedings and the pediatrician cannot pass a nasogastric tube into the stomach (Fig. 4.23).
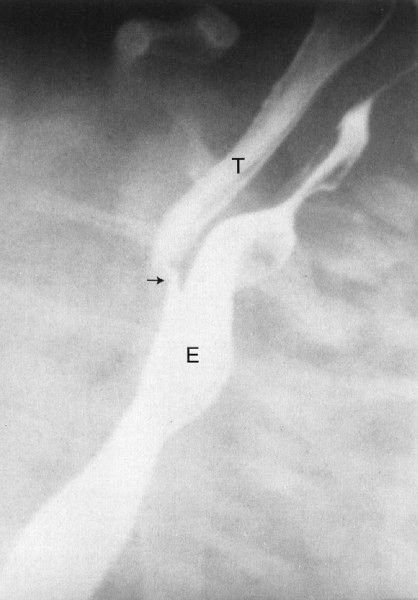
There are two less prevalent but still frequent variants of esophageal abnormalities. The first is esophageal atresia without fistula, in which case the abdomen is gasless because the infant cannot swallow any air to displace the fluid that is in the abdomen in utero (Fig. 4.24). Such infants are usually quite ill and need emergent surgery. The second variant is tracheoesophageal fistula without esophageal atresia. This so-called H-type fistula can be a difficult diagnosis. The nasogastric tube test is normal so that the diagnosis is not readily apparent to the clinician. The child usually presents frequent pneumonias because each time the infant eats, some of the material goes into the lung. Whenever you have an infant with frequent and recurrent pneumonia, this entity, along with cystic fibrosis, needs to be considered (Fig. 4.25). A barium esophagram is necessary to confirm the diagnosis.

FIGURE 4.23 This very ill infant choked on being fed his first bottle. The chest radiograph shows a very dense right upper lobe infiltrate because of aspiration pneumonia. Note that the nasogastric tube won’t go any farther than the upper esophagus (arrows). A surgical clip was placed to identify the site of the tracheoesophageal fistula as this infant was too ill to undergo primary repair of the esophageal atresia; a first-step procedure, ligating and dividing the fistula, was undertaken to protect his lungs. Survival of children with tracheoesophageal fistula correlates directly with the amount of lung disease owing to aspiration.
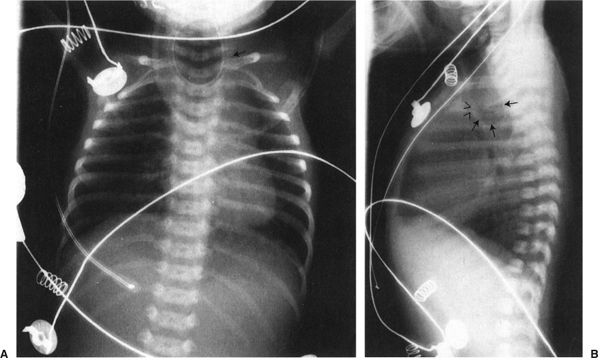
FIGURE 4.24 A: A typical radiograph of a child with esophageal atresia. Note the nasogastric tube (arrow) coiled in the upper esophagus. There are two major differences between this film and that of the child shown in Fig. 4.23. First, this child has not gotten aspiration pneumonia because this abnormality was recognized earlier and the child was not fed. Second, note that there is no gas in the abdomen. Patients with tracheoesophageal fistula always have the very distended stomach as each breath pumps air into that organ. The absence of gas in the abdomen makes the diagnosis of esophageal atresia without fistula. B: This lateral view of the same patient shows the very dilated proximal esophagus (arrows). Note that the dilated esophageal pouch displaces the airway anteriorly (arrowheads). These infants will often have abnormalities of the airway that accompany the abnormalities of the esophagus.
FIGURE 4.25 A barium esophagram on a baby with recurrent pneumonia shows a connection between the esophagus (E) and the trachea (T), a so-called H-type tracheal–esophageal fistula. This abnormality can sometimes be extremely difficult to detect.
CONGENITAL BOWEL ABNORMALITIES
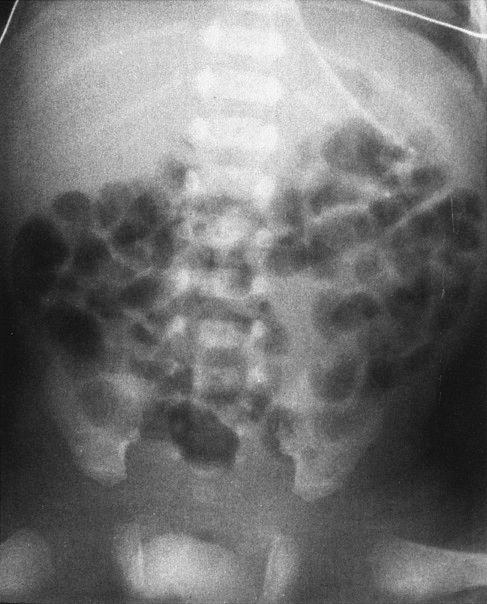
In babies, the commonest cause of bowel obstruction is atresia of the bowel. Atresia occurs owing to a number of complex intrauterine processes, most of which involve vascular supply to the wall of the bowel. Radiographs of the atresia vary tremendously according to the level at which the atresia occurs; however, they have common features. First, there is no gas distal to the level of the atresia, and second, the bowel proximal to the atresia is disproportionately dilated. Beyond that, it is just a matter of looking at the radiograph to try to guess how far down the bowel you can go before you encounter the atresia (Figs. 4.26 to 4.28). As a general rule to help you establish the level, remember that the duodenal bulb is located in the right upper quadrant of the abdomen; therefore, if you have a dilated stomach and loop only in the right upper quadrant, duodenal atresia is likely. The jejunum is predominantly in the upper abdomen and predominantly on the left side, whereas the ileum is in the right lower quadrant. If you see many dilated bowel loops and particularly large loops preponderantly to the right of the spine, it is probably an ileal atresia, whereas if the loops are confined to the upper abdomen and predominantly to the left, it is probably jejunal atresia. These are 70:30 rules, so don’t get too preoccupied with them; on the other hand, they can be very helpful.
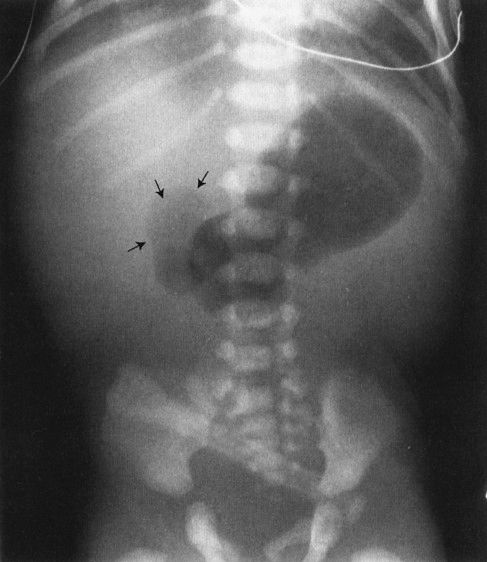
FIGURE 4.26 A newborn infant with marked abdominal distention. Note that the gas goes no farther than the very dilated duodenal bulb (arrows). This is characteristic of the so-called double-bubble sign of duodenal atresia. Whenever you see a patient with duodenal atresia, think of the very frequent associations of Down syndrome and congenital heart disease and the less frequent association of esophageal atresia.
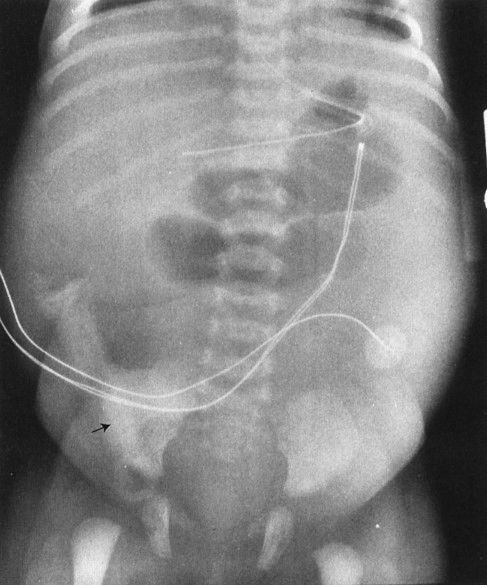
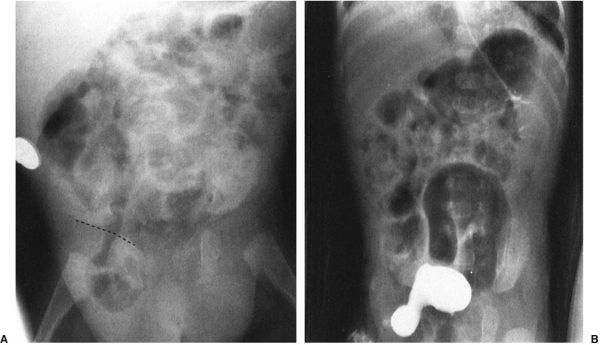
FIGURE 4.27 This infant had a moderate degree of abdominal distention at birth, which progressed to a worrisome abdominal distention within 6 hours. Note that there is no gas in the rectum and that only two dilated loops of bowel are identified, predominantly in the upper abdomen and to the left of the spine. These films are most consistent with abdominal obstruction very high in the bowel but distal to the duodenum. This is an example of surgically proven jejunal atresia.
FIGURE 4.28 A: Abdominal radiograph of a 1-day- old baby with abdominal distention shows evidence of multiple dilated bowel loops and no gas in the rectum. The findings are consistent with an ileal atresia. B: A decubitus view of the same infant illustrated in A shows multiple air–fluid levels. The presence of air–fluid levels is sometimes valuable in distinguishing ileal atresia from meconium ileus. Air–fluid levels favor ileal atresia. Incidentally, note our old friend, the cord clamp.
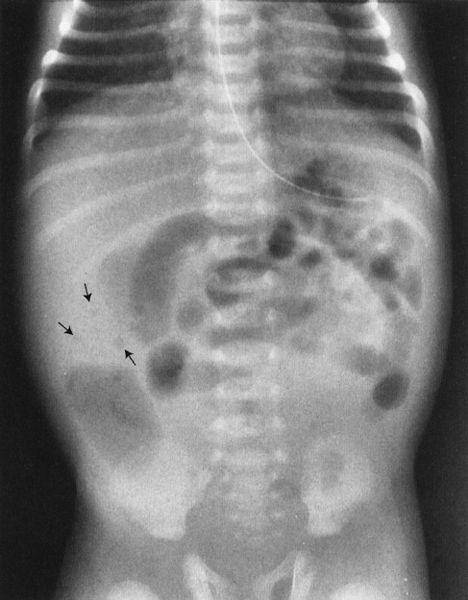
FIGURE 4.29 A newborn infant with a very distended abdomen. Note the bubbly appearance (arrows) in the right lower quadrant of the abdomen. These bubbles coupled with a paucity of gas in the rectum are characteristic of meconium ileus. The vast majority of babies with meconium ileus will also have cystic fibrosis.
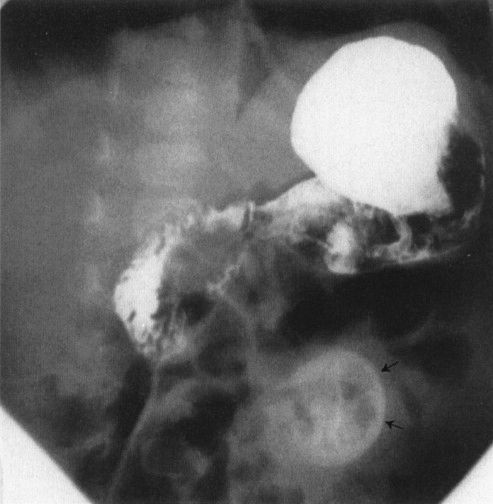
Meconium ileus is a condition that mimics a distal bowel atresia and that deserves special note because of its prevalence in the white population. In meconium ileus, the contents of the bowel (meconium) are abnormal, becoming thick and viscous due to the lack of digestive enzymes. This material compacts in the ileum to cause a complete obstruction. One could think of it as analogous to filling a pipe with tar. Technically, this is not an atresia; however, it mimics an atresia because the bowel is completely obstructed by intraluminal content. There are a few signs that can be used to distinguish meconium ileus from ileal atresia. The meconium ileus usually entraps some air so that one sees a bubbly appearance at the level of the meconium-filled bowel (Fig. 4.29). Also, the meconium is so thick and tarlike that it does not form air–fluid levels with the swallowed intestinal gas. This is in contradistinction to an ileal atresia, whereby the fluid intraluminal contents of the bowel interact with the swallowed gas to form multiple air–fluid levels.
No discussion of intestinal obstruction in neonates would be complete without mention of microcolon. Microcolon is another term for the small lumen colon that is encountered in babies with distal intestinal obstruction, usually either meconium ileus or ileal atresia. Babies’ colons dilate because of the presence of intraluminal content, that is, meconium. This meconium is formed in utero by sloughing of the cells and mucus from the GI tract. If there is enough downstream bowel from an obstruction (e.g., in the instance of duodenal atresia), there will be enough cells and mucus sloughed to form meconium and the colon will have this meconium content. If, however, the obstruction is low so that there is not enough bowel distal to the obstruction to form meconium, then one gets an unused or microcolon. Microcolon is, therefore, a common sign of distal bowel obstruction (Fig. 4.30). You can obviously be fooled, because diseases like Hirschsprung’s disease, in which the lumen is intact but the colon does not transport meconium normally, occasionally give you a microcolon. Again, the 99% rule prevails that microcolon means distal obstruction.
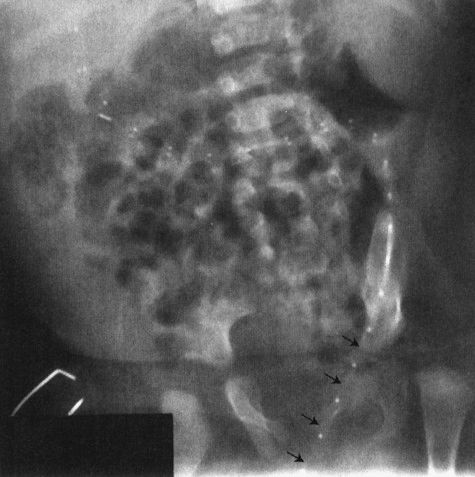
Two other congenital anomalies merit discussion as the cause of abdominal catastrophes in neonates or in slightly older children. The first is the most common cause of bowel obstruction in children, the hernia. Hernias in children are usually congenital defects that allow the bowel to protrude into a space where it doesn’t belong. The bowel then gets caught, swells, and complications ensue. The most common site of hernia is the inguinal area (Fig. 4.31); however, internal hernias, particularly in areas where the bowel goes from retroperitoneum to an intraperitoneal location, are also possible (Fig. 4.32). Hernias can go on for a long time if the bowel does not become compromised, but they can become very symptomatic very fast in instances where the bowel is compromised (Fig. 4.33). Some conditions of the neonate may enhance the possibility of a hernia, particularly conditions that involve chronic ascites, prematurity, or the presence of a ventricular peritoneal shunt for hydrocephalus (Fig. 4.34).
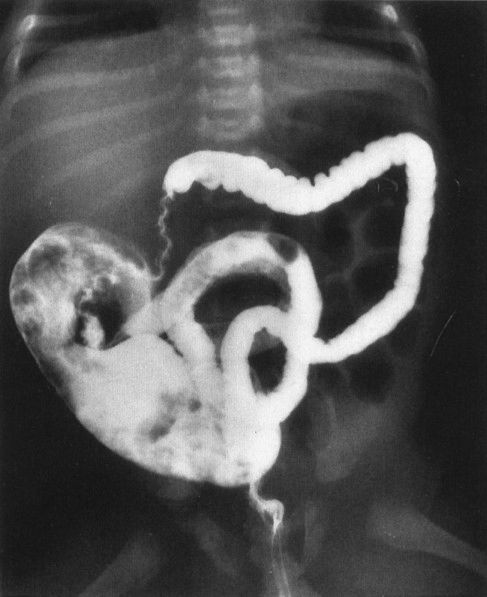
FIGURE 4.30 A contrast enema performed on the patient shown in Fig. 4.29 shows a very small (micro-) colon leading to a very dilated ileum distended with multiple filling defects. The filling defects are the impacted meconium, which gives meconium ileus its name. Remember that a microcolon means a distal obstruction.
FIGURE 4.31 A: Plain radiograph of the abdomen shows bowel lying below the inguinal ligament (dotted line). On a plain radiograph, the inguinal ligament is defined as the space between the symphysis pubis and the anterior superior iliac spine—in this case, the child had a large inguinal hernia filled with bowel. B: This baby, having a contrast study of the kidneys, had a protrusion of the lateral margin of his bladder into the right inguinal canal. A small protrusion can be normal; however, this large protrusion of bladder is associated with an inguinal hernia.

FIGURE 4.32 This neonate has a large hiatus hernia or protrusion of stomach above the diaphragm. The hernia pouch (arrows) is shown in the chest, whereas the narrowing of the esophageal hiatus (arrowheads) shows the area where the stomach herniates through the periesophageal hiatus. Note that there is contrast in the lungs. This hernia was so large that it caused the baby to reflux contrast from his stomach to his esophagus and then aspirate. Unlike this one, most hiatus hernias are relatively benign.

FIGURE 4.33 This premature infant developed signs of a bowel obstruction. Note the very dilated loops of small bowel on the film. The most important finding is the asymmetry of the inguinal folds, the right bulging in a concave fashion (arrowheads), whereas the left is straight (arrows). The bulge in the right groin is owing to an incarcerated inguinal hernia, a finding the clinicians missed until they took off the patient’s diaper.
FIGURE 4.34 This baby with hydrocephalus had a ventricular peritoneal shunt. The radiopaque markers (arrows) show the course of the shunt. Notice that the shunt extends into an inguinal hernia. Because these babies have chronic ascites from the drainage of the cerebrospinal fluid into the abdomen, they are more prone to have inguinal hernias. Approximately one third of babies with ventricular peritoneal shunts develop inguinal hernias.
The other congenital abnormality worthy of special attention is that of malrotation. This condition occurs because of a congenital abnormality of fixation of the bowel. Remember that the bowel forms about the axis of the superior mesenteric artery (I bet you never thought you’d ever need that bit of embryology) and that the bowel herniates out of the body through the omphalus, then returns to the abdominal cavity. If, on return, the bowel does not rotate appropriately, it fixes in abnormal positions. This error in fixation of the bowel sets the scene for the bowel to twist and obstruct, causing the so-called midgut volvulus (Figs. 4.35 and 4.36). This is truly a surgical emergency and should be considered whenever you have an abnormal film suggesting obstruction as well as the presence of bilious vomiting in a child. Because the bowel twists about the superior mesenteric artery and vein, the major complication is vascular compromise of the bowel. If the bowel is not untwisted, the gut will die, leaving the child a nutritional cripple. Although malrotation is discussed with the neonatal diseases, be aware that it can present at any time in life. The majority of malrotation patients who get in trouble do so before 2 years of age; however, older children and adults can occasionally have malrotation-related problems.
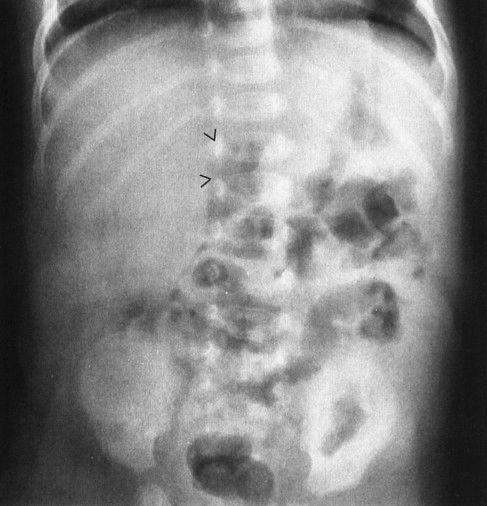
FIGURE 4.35 This 36-day-old infant began vomiting bilious material and became acutely ill. The plain abdominal radiograph is not diagnostic; however, note the gas-filled duodenal bulb (arrowheads). It is very unusual to see gas in a normal duodenal bulb. The subsequent upper GI proved that this patient had a midgut volvulus. The plain film findings in midgut volvulus range from normal to complete bowel obstruction. When in doubt with an infant with bilious vomiting, note that an upper GI is always indicated.
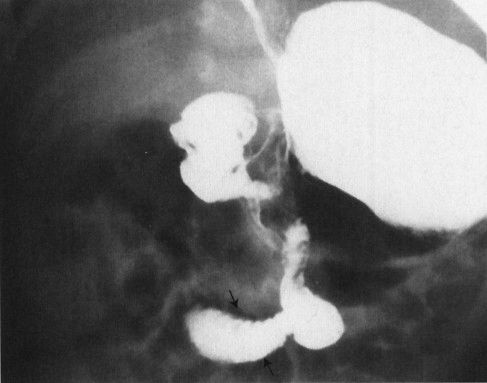
FIGURE 4.36 Upper GI in a 27-day-old baby who suddenly developed bilious vomiting. Note that the duodenum descends in the midline, then passes off to the right at the duodenal–jejunal junction (arrows), never coming to the left of the spine and behind the stomach as is normal. This is characteristic of malrotation, an anomaly that occurs due to malfixation of the gut in utero. The greatest danger with these infants is midgut volvulus and infarction of the small bowel because of twisting about the vascular pedicle at the root of the mesentery.
FIGURE 4.37 A: This child’s heart measures over 60% of the transverse diameter of the chest on the radiograph. In an adult, this would be a large heart; however, this is a normal child with a large thymus simulating cardiomegaly. B: Note that on lateral view, the heart does not protrude posterior to the airway (arrows).
Serious congenital heart disease has a prevalence of approximately 1 per 1000 live-born infants and, therefore, is a disease that you will likely encounter if you care for children. You could argue that this belongs in the neonatal section, but many children with serious heart disease present later, so I left it here—life is imperfect!
Although plain film is valuable for screening for congenital heart disease, it takes a tremendous amount of experience (and luck) before you can be specific as to the type of congenital heart disease. Several principles are very important. The first is that heart size in infants and children is more difficult to estimate than that in adults. The rule of thumb of 50% cardiothoracic ratio is not valid in children. When you’re looking for cardiomegaly in a child, you need to be sure that you are not looking at thymus, that the film has been taken on a good breath, and that lateral views are used extensively. If the heart protrudes significantly beyond the visible airway on lateral view, the heart is usually enlarged. If on an anteroposterior view the heart appears large whereas on the lateral view it is normal, then you are usually dealing with a deceiving thymus (Fig. 4.37).
The second rule is that children can have very serious heart disease and a normal-sized heart. This is particularly true in conditions in which blood flow to the lungs is insufficient because of right-to-left shunting. As a good general rule, children’s hearts, being resilient, tend to dilate owing to a volume rather than a pressure overload. Conditions that cause right-to-left shunting, like tetralogy of Fallot, do not give you an enlarged heart because the volume of blood traversing the heart is actually diminished (Fig. 4.38). A truly cyanotic neonate with a normal chest film (including heart size) usually has some variant of tetralogy of Fallot.
Rule number three is that if you think that the pulmonary vascularity is increased in a patient suspect for congenital heart disease, you are probably right; however, if you think it is decreased, you are probably wrong. For some reason, it is much easier for humans to perceive an increase in vascularity than a decrease in vascularity in a chest film, probably because of the way our brains are wired. An enlarged heart and increased vascularity in an older child who is not cyanotic usually mean some form of left-to-right shunt such as a ventricular septal defect (Fig. 4.39).
Rule number four is applicable to neonates. We have already talked about the fact that being born is the ultimate time of transition. When you are in utero, very little blood goes through the pulmonary artery circuit. To understand this, think about physiology. In utero, the baby is not breathing air; therefore, there is no need for blood to bring oxygen to the baby from the lungs. Immediately following birth, the baby breathes air and the situation changes dramatically. It takes some time for the pulmonary arterial flow to reach adult levels of blood flow through the lungs. All neonates, therefore, have a relative state of pulmonary hypertension. Early on, lesions that should have increased vascularity, such as transposition of the great vessels, are not revealed by radiograph (Fig. 4.40). The same logic explains why most left-to-right shunt lesions are not manifest until about 6 weeks of age (see rule three) when the pulmonary artery pressure has fallen significantly.
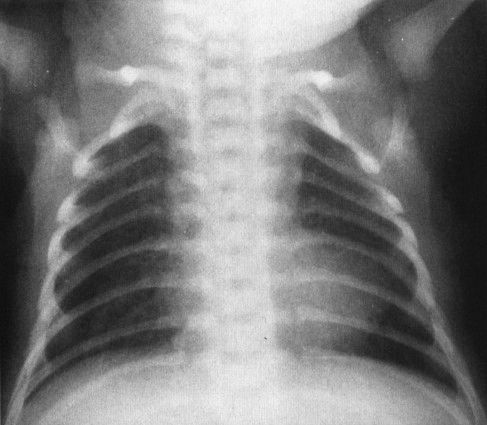
FIGURE 4.38 This cyanotic, extremely ill child has a relatively small but boot-shaped heart. The pulmonary vascularity is normal because of the presence of a patent ductus arteriosis. The findings are characteristic of a variant of tetralogy of Fallot.
FIGURE 4.39 The anteroposterior (A) and lateral (B) radiographs of a 2-month-old infant with respiratory distress when feeding, but no evidence of cyanosis, show a markedly increased pulmonary vascularity and a large heart. This combination in a child is characteristic of a congenital left-to-right shunt. The most common left-to-right shunt is a ventricular septal defect or a communication between the right and left ventricles.

FIGURE 4.40 This very cyanotic patient has a chest radiograph showing a large heart, narrow superior mediastinum, and pulmonary vascularity that is slightly, but not dramatically, increased. The patient is a 3-day-old infant with transposition of the great vessels; the vascularity is going through the transition between the very high vascular resistance in utero and the low vascular resistance of an air-breathing baby. Over the course of subsequent days, the vascular resistance will drop further and the lungs will become flooded.
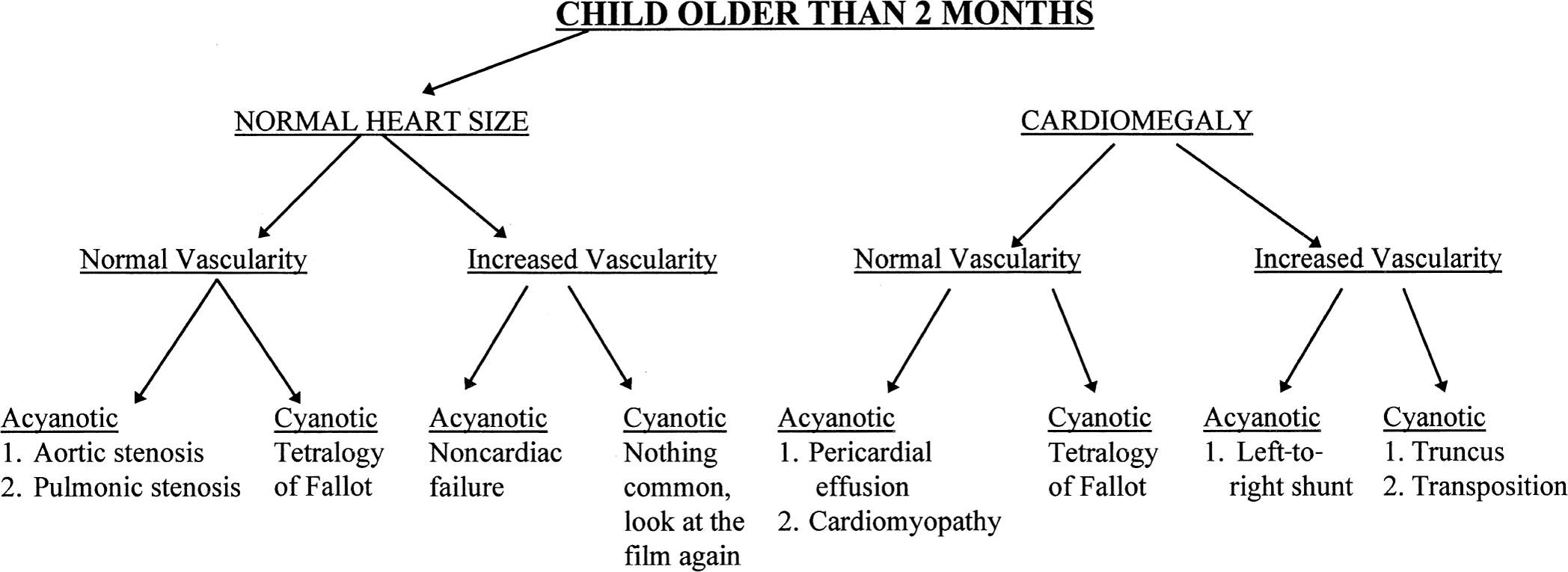
With those rules to consider, it is possible to set out a systematic approach to looking at the chest film of a newborn with congenital heart disease. First, see whether the heart is enlarged and whether you can determine which chamber is enlarged. Next, determine whether the vascularity is normal or increased, keeping in mind that the younger the baby, the less confident you can be to find increased vascularity. Finally, you need to talk to your clinical colleagues and find out whether the baby is truly cyanotic, as defined by arterial oxygen saturation of under 80% with a normal arterial CO2 saturation. With those pieces of information you can look at Figs. 4.41 and 4.42 and make a rough estimate as to what type of congenital heart disease the baby may have (Figs. 4.43 to 4.45).
Other than the thymus and bronchi, the remainder of the structures in the pediatric mediastinum are relatively inconspicuous unless abnormal. Pediatric mediastinal abnormalities (masses) conform to a compartmental scheme. If you can accurately identify the compartment where a mass is located, you can provide an intelligent differential diagnosis. The anterior mediastinum is defined as part of the mediastinum visible in front of the airway on lateral view, and the posterior mediastinum is defined as that portion of the mediastinum just posterior to the anterior edge of the vertebral bodies on lateral view. Everything else is the middle mediastinal compartment (Fig. 4.46). The whole trick is telling these compartments apart, and there are a few rules:
1. Clavicle cutoff sign: The anterior chest is anatomically lower than the posterior chest, so if a mass stops at the inferior margin of the clavicle on the PA chest radiograph, it has to be in the anterior mediastinum (Fig. 4.47).
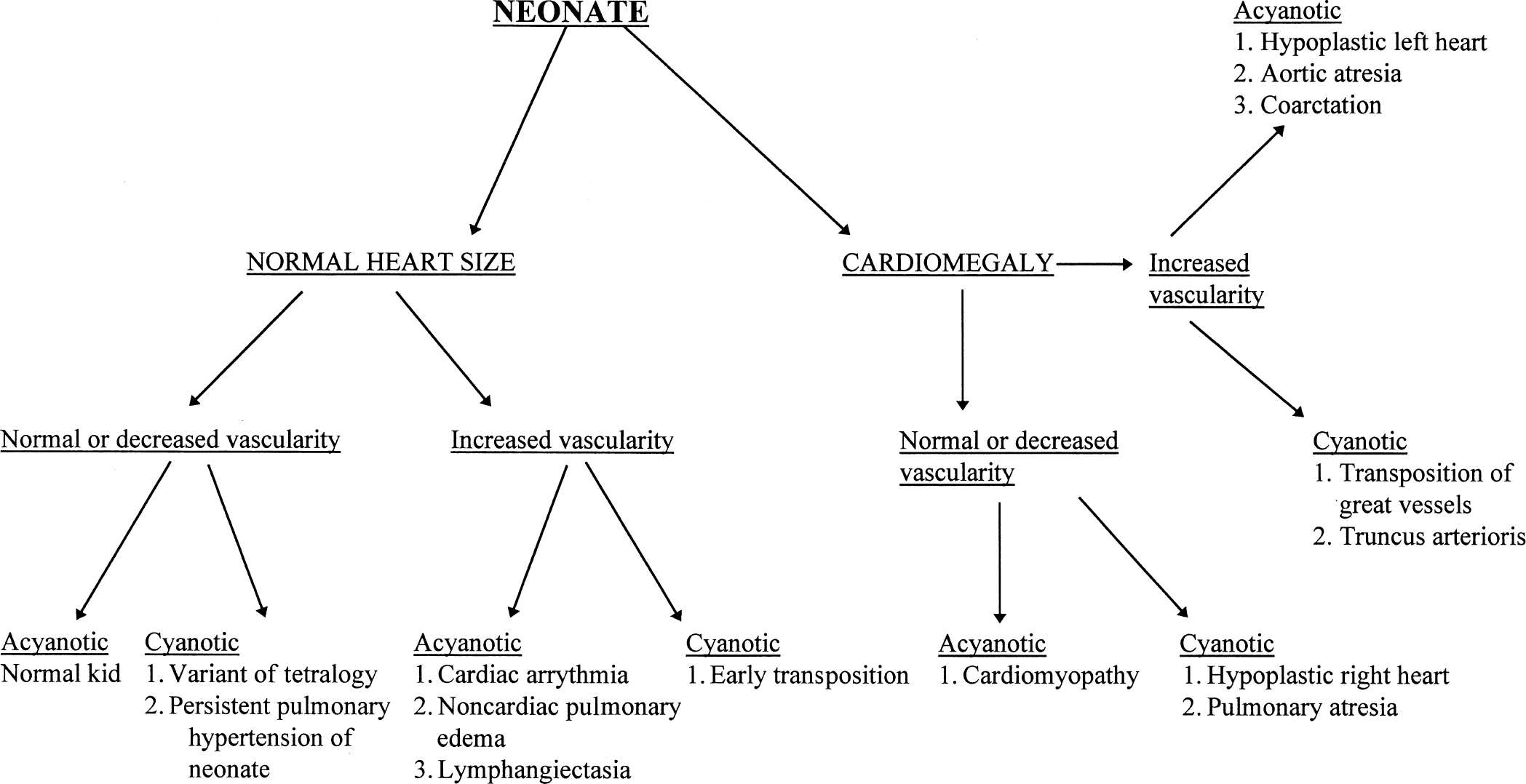
FIGURE 4.41 Flow diagram for neonates suspect for heart disease.
FIGURE 4.42 Flow diagram for older children suspect for heart disease.

FIGURE 4.43 This child has a huge heart. Note that the superior mediastinum has a large shadow to the right of the trachea, a right aortic arch. The vessels are large, flooding the lung. If we add the information that the child was cyanotic, then this becomes a characteristic truncus arteriosis. Approximately 40% of truncus patients have a right arch.
FIGURE 4.44 A large heart and increased vascularity; however, this time the child is not cyanotic. This usually means either a left heart obstructive lesion or a failure for some other reason. The child has a hypoplastic left heart.
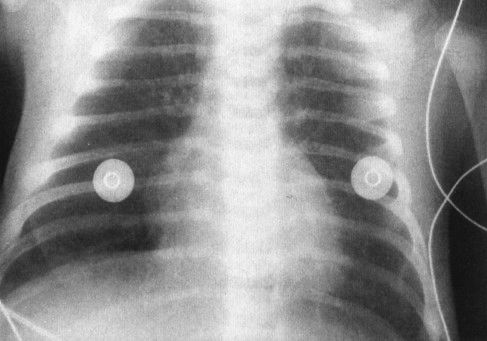
FIGURE 4.45 A large heart and increased vascularity in a 2-week-old baby—findings typical for transposition of the great vessels. Note the very thin mediastinum because the pulmonary artery is directly in front of the aorta as opposed to being slightly to the left of it. This is characteristic of the transposition of the great vessels.
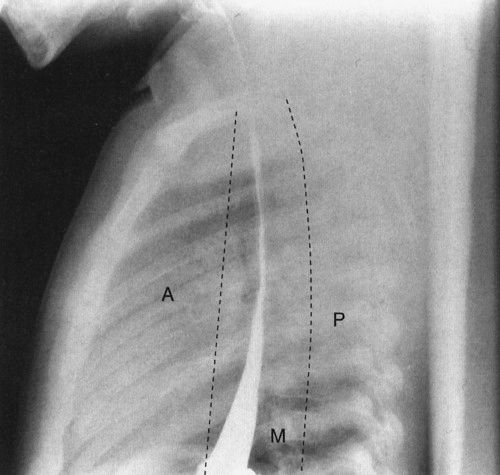
FIGURE 4.46 A normal lateral view of the chest, with barium in the esophagus, delineates the boundaries of the anterior (A), middle (M), and posterior (P) mediastinum. When you consider mediastinal masses, it is important mentally to divide the mediastinum into these components as it helps you localize the likely diagnosis for the cause of the mass.
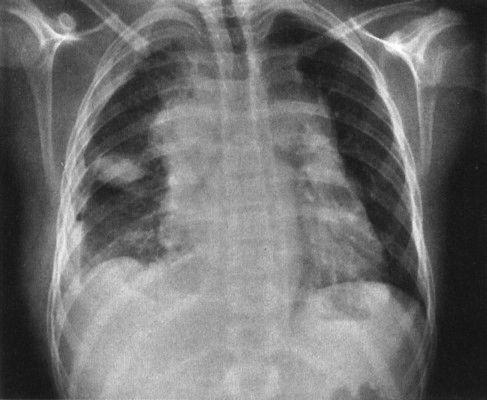
FIGURE 4.47 This anterior mediastinal lymphoma stops right at the undersurface of the clavicles. Remember that the anterior portion of the chest is physically lower than the posterior portion of the chest; therefore, any mass that has the clavicle as its superior margin must be an anterior mediastinal mass.
FIGURE 4.48 This teenager has an anterior mediastinal mass. Note that the descending branch of the right pulmonary artery (arrows) is visible through the mass, documenting that the tumor is not in the same plane as the vessel; otherwise, the silhouette sign would prevent the vessel from being visible. This is called the hilum overlay sign where masses out of plane of the hilum (usually anterior mediastinal masses) allow hilar structures to be visualized.
2. Hilum overlay sign: Structures in the far anterior mediastinum overlie the vessels at the lung hilum; therefore, the vessels are usually seen through these structures (Fig. 4.48).
3. Posterior rib effacement: Posterior mediastinal masses frequently spread the posterior ribs; therefore, distortion or asymmetry of the posterior ribs is a good sign that the mass is posterior (Fig. 4.49).
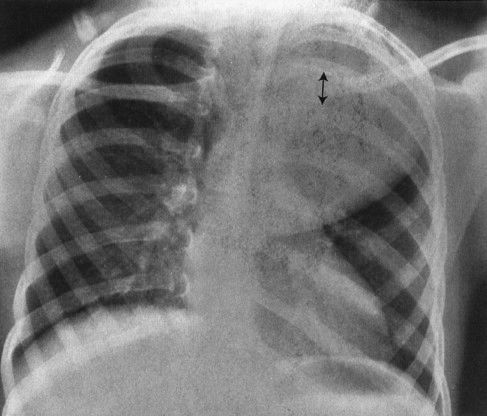
FIGURE 4.49 This child has a huge posterior mediastinal mass on the left. Note how the mass has spread the ribs posteriorly. Masses that distort the posterior ribs are almost always neural crest in origin, in this case a ganglioneuroblastoma.
4. Airway distortion sign: Masses that distort the esophagus or compress the airways are almost surely middle mediastinum (Fig. 4.50).
Once you have applied these rules and decided in which compartment to look, the pathologic processes tend to categorize themselves fairly easily. Anterior mediastinal masses are almost always lymphoma or thymus related with the occasional thyroid mass or teratoma. An Aunt Minnie applies here: If the anterior mediastinal mass contains calcium, always go for teratoma (Fig. 4.51). Middle mediastinal masses are generally either lymph nodes or anomalous vessels related to the aortic arch. Esophageal and bronchial duplications are less frequent but also occur in the middle mediastinum. Posterior mediastinum masses are neurogenic in origin, usually a neuroblastoma or ganglioneuroblastoma. When confronted with a suspected pediatric mediastinal mass, the first rule is to place it in the proper compartment; thereafter, it is a matter of pursuing the differential diagnosis.
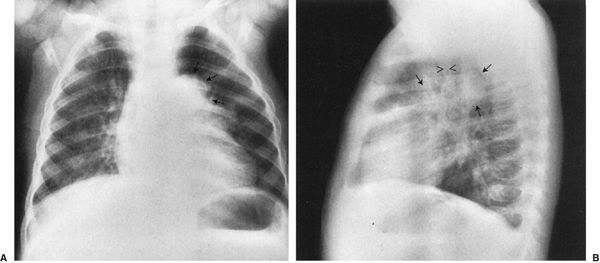
FIGURE 4.50 A: The large mass (arrows) near the mediastinum is displacing the upper lobe bronchus on the left. Note that the left lung is blacker than the right because the mass is causing partial obstruction of the left main stem bronchus, allowing air to get in but not to escape easily. The effect on the bronchus or blood vessels is characteristic of a middle mediastinum mass, in this case, a bronchogenic cyst. B: Lateral view shows the rounded mass (arrows) of the bronchogenic cyst in the same plane as the airway (arrows).
FIGURE 4.51 The mass lying anterior to the right hilum (arrowheads) contains a large glob of high-density calcium. Remember that anterior mediastinal mass plus calcium equals teratoma.
A common intra-abdominal condition worth discussion in babies (not brand new but 4 to 6 weeks old) is pyloric stenosis, which isn’t a congenital anomaly. Pyloric stenosis is due to hypertrophy of the pyloric muscle induced by a heritable error in metabolism. Because of the heritable nature of the disease, it is somewhat a congenital defect, but it does not usually present right after birth because it takes some time for the abnormal chemistry to cause the pyloric muscle to hypertrophy to a sufficient degree to obstruct gastric outflow. The disease is male preponderant and classically presents with nonbilious vomiting and weight loss in a 6-week-old infant. The plain abdominal radiograph suggests a partial obstruction with a very dilated stomach. Upper GI will show elongation of the pyloric channel and the narrowing of that channel. Ultrasound is the current favored method for the definitive diagnosis, showing the very large pyloric muscle tumor in exquisite detail (Figs. 4.52 to 4.54).
CONDITIONS IN OLDER CHILDREN WITH CYSTIC FIBROSIS
When you are studying pediatric patients, it is always important to remember that the prevalence of congenital or heritable abnormalities is higher among the pediatric population than the adult population. Therefore, in looking at the radiographs of a child with recurrent pneumonias, you should think about heritable conditions that predispose the child to pneumonia, an example being cystic fibrosis. Cystic fibrosis, the most prevalent lethal genetic disease among the white population, begins with recurrent pneumonias but also has a number of features that allow a specific diagnosis from the films (Fig. 4.55). The lungs are usually hyperexpanded because of the blockage of many of the smaller bronchi by mucous plugs. The presence of mucoid impactions, branch-shaped collections of intrabronchial mucus, is very suggestive of cystic fibrosis. Generally, the children have very prominent hili due to the combination of the inflamed lymph nodes and pulmonary artery enlargement resulting from pulmonary hypertension caused by lung destruction. The last common finding of cystic fibrosis is that of peribronchial cuffing, or thickening of the walls of the bronchus, due to the intense inflammatory change induced by the disease (Fig. 4.56). None of these signs are pathognomonic for cystic fibrosis; however, all of these signs taken in combination make the likelihood of this disease very high.
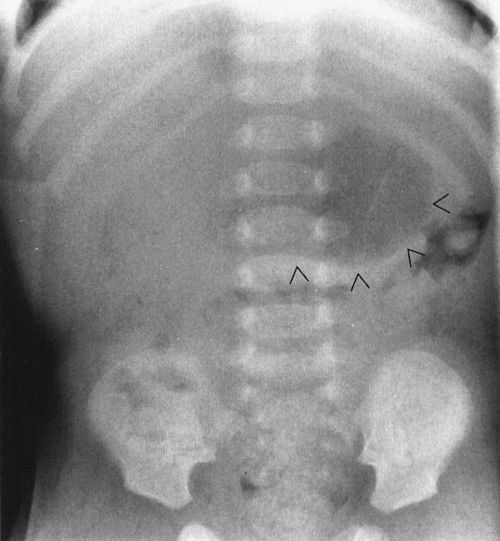
FIGURE 4.52 This 6-week-old infant had severe and pernicious vomiting that had become progressively worse over the course of a week. The impact on his nutrition was so severe that he had actually lost weight. Note the distended stomach (arrowheads). The presence of distal gas in his rectum shows that he was not completely obstructed, but the film is suggestive of a high-grade partial gastric outlet obstruction. About 90% of pyloric stenosis patients will have a plain film that looks like this.

FIGURE 4.53 The upper GI in this 6-week-old baby shows elongation and narrowing of the pyloric channel (arrowheads). On the stomach side, notice the rounded indentation (arrows) caused by the very hypertrophied pyloric muscle. This is called the shoulder sign. Together this combination of signs is diagnostic for pyloric stenosis.
FIGURE 4.54 A: A longitudinal ultrasound view of the pylorus in a 6-week-old boy with vomiting. The pyloric muscle appears black, whereas the mucosa of the central lumen appears white (arrows). During the entire period of observation, the configuration of the pyloric muscle did not change and measurement of the pyloric muscle revealed a thickness of 6 mm (normal is greater than 4 mm). This is diagnostic of pyloric stenosis. Note the intimate relationship of the pylorus to the right kidney (K). B: Transverse ultrasound view of the pylorus in the same infant as shown in A. Again the black or hypoechoic muscle surrounds the very echogenic mucosa. Arrows outline the transverse view of the pylorus. In pyloric stenosis, the muscle is thick and unchanging throughout the examination.
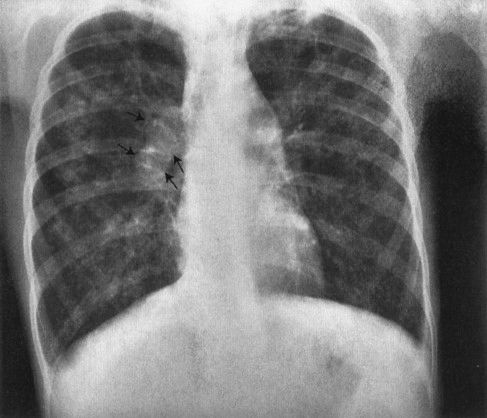
FIGURE 4.55 Typical chest film of a child with cystic fibrosis. Patchy infiltrates are present in both lung fields. The right hilus is very large and irregular owing to a combination of enlarged pulmonary artery and lymph nodes (arrows). Also note that the heart is very small because of markedly overly expanded lungs. The pulmonary arteries are large because of a pulmonary hypertension. This is a very typical appearance for cystic fibrosis.

FIGURE 4.56 A close-up of the lung showing peribronchiolar thickening. Note that the bronchus appears as a black dot amid a white cuff. This cuff is edema and inflammatory cage in the bronchial wall.
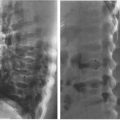
Intussusception, a disease in which one segment of bowel telescopes into another, has its maximum prevalence between the ages of 6 months and 2 years. The bowel is constantly in motion because of normal peristaltic activity. Theoretically, an inflamed intramural lymph node or some other structure alters this peristaltic activity, such that one segment of bowel begins to be propelled at a differential rate, leading to prolapse of one segment (intussusceptum) into the next contiguous portion of bowel (intussuscipiens). The intussusceptum becomes edematous because the blood supply of the prolapsed bowel is compromised and the intussusceptum begins to swell. This compounds the problem and leads to further extension of the intussusception. The ultimate extension is protrusion of the intussusceptum from the rectum! In fact, in nineteenth century textbooks, the differential diagnosis of intussusception was rectal prolapse. The most common anatomic area involved in intussusception is the terminal ileum, and most intussusceptions are ileocecal.
Radiology plays a key role in the diagnosis as the child often presents with acute abdomen. Plain film shows evidence of partial bowel obstruction, and the intussusceptum is frequently visible on the plain film as a rounded density near the point of obstruction (Fig. 4.57). Diagnosis is confirmed either by ultrasound or by barium enema (Figs. 4.58 and 4.59). Radiology often plays both a diagnostic and a therapeutic role in intussusception. Between 50% and 80% of intussusceptions can be nonoperatively reduced using either an air enema or a contrast enema. These techniques are very specialized and should be performed only by trained personnel. They are, however, part of the standard armamentarium of any board-certified radiologist.
Appendicitis is the most common abdominal surgical emergency of later childhood. Appendicitis is one of the great mimics in that the symptoms can be fairly protean and the diagnosis in many instances is obscure (Table 4.3). There is an old adage that the clinical certainty of appendicitis should be about 85%, meaning that 15% of the appendices that a surgeon removes should be normal or the surgeon is going to begin to miss some true-positive cases of appendicitis. Various radiology imaging techniques can narrow this false-positive rate somewhat, but really the value of imaging is in those cases in which the diagnosis is in serious doubt. Look at it in another way. If imaging tests can give you 95% sensitivity, whereas clinical tests are 85% sensitive, the imaging doesn’t add much in a situation in which you are quite sure that appendicitis exists. On the other hand, in that case when you aren’t sure or, let’s say, you are only 30% sure, a 95% sensitivity test is crucial in deciding appropriate patient management. At current hospital prices, it is cheaper to do the test than to put the patient in the hospital overnight for observation. Appendicitis is discussed in detail in Chapter 3, so it will only be mentioned here.

FIGURE 4.57 Notice the rounded density and a spiral gas pattern located in the right upper quadrant in this 2-year-old child with recurrent cramping, abdominal pain, and hematochezia (arrows). This plain film alone, if not diagnostic, is certainly very suspicious of intussusception. About 80% of the time you can make the diagnosis of intussusception on plain film by looking for a rounded mass and the spiral air pattern that is so characteristic.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree