Fig. 20.1
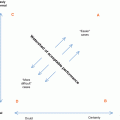
Skull radiograph of an 18-month-old child who suffered non-accidental injury shows multiple skull fractures (open arrows) with the straight sharp margins. This contrasts with the zigzag pattern of unfused sutures with interdigitations and sclerotic margins (arrows)
20.2.2 Cranial Ultrasound Imaging
MRI is considered to be the gold standard for imaging the brain at all ages, including neonates. However, many of these neonates are too sick to be transported for an MRI examination and cranial US imaging is performed in the preterm and term neonates on a routine basis. Familiarity with normal anatomy, variants, and artifacts is essential for correct interpretation (Enriquez et al. 2003).
20.2.2.1 Ventricular System
Asymmetry of the lateral ventricles is a common finding in newborns with a reported frequency of 77 % in normal neonates (Fig. 20.2). Most of the time, the posterior part of the left posterior horn is more prominent compared to the right. Features suggesting that this is a normal variant rather than pathology are the preservation of the triangular configuration of the frontal horns of the lateral ventricles, the thin ventricular walls, and the stable appearance over time. Coarctation of the lateral ventricles or connatal cysts is caused by focal approximation of the walls of the lateral ventricles (Fig. 20.3). They are considered a normal variant as this finding is unrelated to any pathological sequelae. Connatal cysts resolve at 1–2 months of corrected age. These cysts lie at or just below the superolateral margins of the frontal horn or body of the lateral ventricle and anterior to the foramen of Monro. The important differential diagnosis is periventricular leukomalacia (PVL), which are seen above the superolateral margins of the frontal horn and adjacent to the body of the lateral ventricles. PVL is caused by hypoxic-ischemic injury at the watershed territory of the premature infant and is located at the periventricular white matter at the border zone between the deep and superficial arterial vascular distribution. PVL is known for its poor prognosis with development of cerebral palsy, intellectual impairment, and visual disturbances. In particular, the presence of bilateral parieto-occipital cysts is highly predictive for the development of cerebral palsy (Epelman et al. 2006).
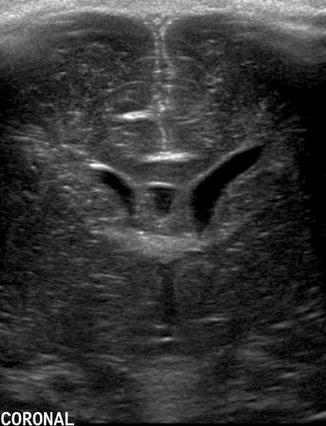
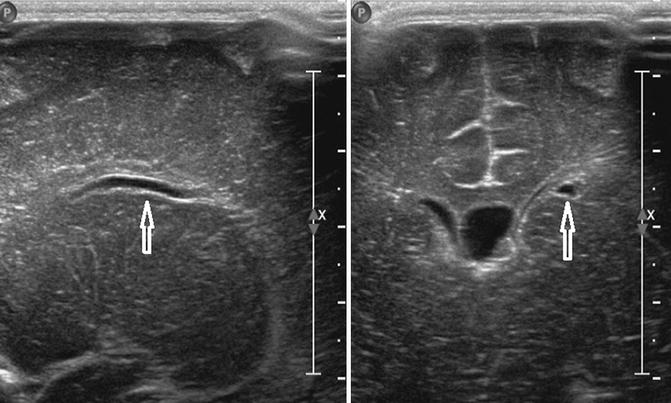

Fig. 20.2
Coronal US image taken through the anterior fontanelle at the foramen of Monro shows asymmetry of the lateral ventricles with the left side being wider than the right. The triangular appearance is preserved and the ventricular walls are thin, indicating that this finding represents a normal variant

Fig. 20.3
Coronal (right) and parasagittal (left) US images show a connatal cyst (arrowed) just below the superolateral margins of the left frontal horn of the lateral ventricle
Other well-known normal variants are the cavum septum pellucidum and cavum vergae (Fig. 20.4a, b). The cavum septum pellucidum is a fluid-filled space situated between the two leaves of the septum pellucidum, extending from the corpus callosum to the columns of the fornices. The cavum vergae is a fluid-filled space between the two leaves of the septum pellucidum, posterior to the vertical plane formed by the columns of the fornices. The cavum septum pellucidum and cavum vergae communicate with each other and obliterate from posterior to anterior. The more premature the newborn, the more commonly these are seen, but they can persist until adulthood. An enlarged cavum septum pellucidum has been described in several neuropsychiatric and post-traumatic disorders. The cavum velum interpositum is less frequently seen. It is located at the pineal region below the column of the fornices, above the internal cerebral vein. By using color Doppler US imaging, a cavum velum interpositum is easily differentiated from a vein of Galen malformation. To distinguish a pineal cyst from a cavum velum interpositum, the position relative to the internal cerebral vein must be discerned. A pineal cyst is situated below the internal cerebral vein.
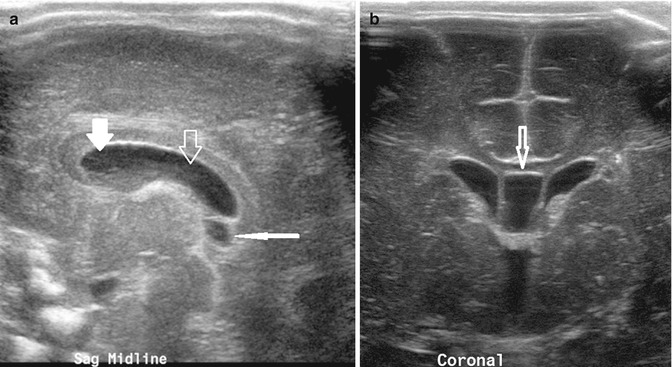

Fig. 20.4
(a) Midline sagittal US image taken through the anterior fontanelle shows a cavum septum pellucidum (thick white arrow), cavum vergae (thick open arrow), and cavum interpositum (thin white arrow). (b) Coronal US image shows the cavum septum interpositum (arrow) situated between the frontal horns of the lateral ventricles
20.2.2.2 Choroid Plexus
Occasionally, a normal choroid plexus can have a lobular appearance that could mimic germinal matrix hemorrhage, indicating a grade II hemorrhage (Di Salvo 2001). Color Doppler US imaging can easily differentiate between these two entities, as the choroid plexus is highly vascular in contrast to the avascular appearance of hemorrhage.
20.2.2.3 Brain Parenchyma
Peritrigonal echogenicity needs to be differentiated from PVL. Peritrigonal echogenicity is caused by an anisotropic effect of the US beam. PVL is suspected if the area of hyperechogenicity is asymmetric, coarse, globular, or more hyperechoic when compared to the choroid plexus. This hyperechogenicity persists in scans through the posterior fontanelle. Mineralizing vasculopathy is seen as increased linear echogenicities within the basal ganglia, related to the thalamostriate and lenticulostriate arteries (Fig. 20.5). The underlying etiology and clinical implications of mineralizing vasculopathy are still under debate. It is idiopathic in most cases, but it has been suggested that this finding is a consequence of diffuse perinatal insults such as TORCHS (Toxoplasmosis, Rubella, Cytomegalovirus, Herpes Simplex) virus infections, trisomies, and ischemia. However, there is no apparent change in outcome in newborns with mineralizing vasculopathy, as compared to matched controls (Makhoul et al. 2003).
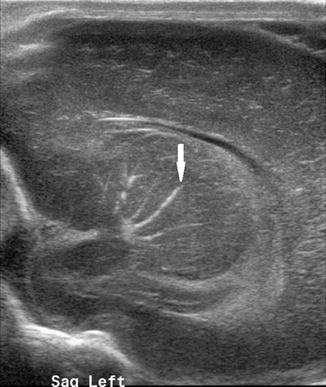

Fig. 20.5
Left parasagittal US image taken through the anterior fontanelle shows linear branching echogenicities within the basal ganglia/thalamus consistent with mineralizing vasculopathy (arrow)
20.2.2.4 Severe Hypoxic-Ischemic Injury
Severe hypoxic-ischemic injury (HII) in term neonates can be easily missed on US examination, because the findings are subtle, generalized, and symmetrical. The abnormal US features seen in neonatal HII are more easily detected when compared to the normal appearance of a neonatal brain on high-resolution US scanning (Fig. 20.6). In HII, there is an increase in the gray-white matter differentiation caused by the white matter becoming more hyperechoic in contrast to the hypoechoic gray matter. However, this is a nonspecific sign and can also be seen in cerebral infections and leukodystrophies. In more severe HII, the appearances are more heterogeneous with loss of gray-white matter differentiation and loss of definition of anatomical landmarks, including the fissures and basal ganglia (Daneman 2009).
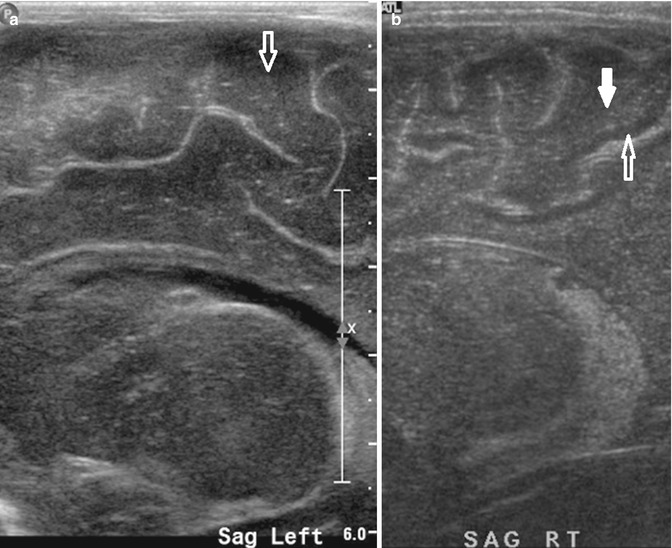

Fig. 20.6
(a) Left parasagittal US image shows normal gray-white matter echogenicity in a full-term newborn. There is little gray-white matter differentiation in the gyri in a normal newborn brain (open arrow). (b) Right parasagittal US image shows abnormal increased gray-white matter differentiation in a full-term newborn with hypoxic-ischemic encephalopathy. There is an increase in white matter echogenicity (white solid arrow) compared to the gray matter (white open arrow), resulting in an increase in the gray-white matter differentiation
20.2.3 Spine Ultrasound Imaging
US is a valuable tool for imaging the spine in newborns and young infants. It is used to screen for suspected congenital malformations such as spinal dysraphism and the tethered cord. There are several normal variants that may simulate disease and these may be potential pitfalls during spinal US (Lowe et al. 2007).
20.2.3.1 Technique-Related Pitfalls
The US examination is performed with high-frequency linear and curved array transducers in the sagittal and the transverse planes. The image quality decreases after 5 months of age because the posterior spinous elements begin to ossify. After 6 months of age, the diagnostic yield is limited and paramedian scans are suggested in such cases. However, in children with posterior spinal defects, the persistent acoustic window enables US to be performed at any age. The vertebral level is determined by counting up from the L5–S1 junction and counting down from the 12th rib. If the level remains unclear, correlation with a spine radiograph with a radiopaque marker placed at the T12 vertebral body may be necessary.
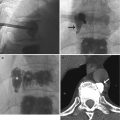
It is important to accurately determine the level where the conus medullaris terminates because an abnormally low-lying cord may imply cord tethering. In term infants, the tip of the conus medullaris normally lies above the level of mid-L2 vertebral body, although there is a large normal range of the position in healthy infants (T10/11–L4) (Wolf et al. 1992). In preterm infants, the conus normally lies between L2 and L4 vertebral levels. Follow-up US examination should be performed to determine the level of the spinal cord at the corrected term gestational age. Searching for other features of tethering, such as the posterior position of the cord within the spinal canal, the absence of the normal pulsations of the spinal cord and nerve roots, an increased thickness of the filum terminale (normal <2 mm), and the presence of a spinal cord mass such as a lipoma, are also important in establishing the diagnosis. MRI is performed for suspicious cases.
20.2.3.2 Normal Variants That May Simulate Diseases
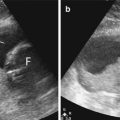
Slight dilatation of the distal central canal can be seen in newborns and this disappears most of the time during the first weeks after birth (Unsinn et al. 2000) (Fig. 20.7). The ventriculus terminalis is a small cystic structure which is located at the transition of the conus medullaris to filum terminale. It originates from an incomplete regression of the embryonic terminal ventricle. It regresses in size during the first weeks of life. This finding is not associated with clinical symptoms. Filar cysts are located within the filum terminale just below the conus medullaris. Similar to the ventriculus terminalis, this should have no complex features on US (Fig. 20.8). The exact origin is uncertain, but this finding has no clinical implications. No further imaging is therefore necessary. A prominent hyperechoic filum terminale can be differentiated from a fatty filum terminale by its midline position and thickness, which should not exceed the normal limits (<2 mm). Nerve root clumping in the decubitus position may stimulate a mass. Rescanning in the prone position will cause the presumed mass to disappear. The pseudosinus tract is a residual cord-like structure that extends from the skin dimple to the coccyx and is a clinically benign finding (Fig. 20.9). True dermal sinus tracts are typically found in a more cranial position and may communicate with the spinal canal.
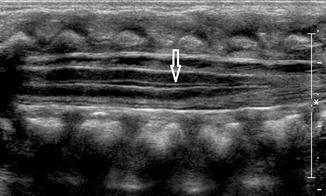
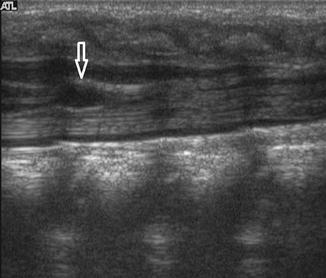
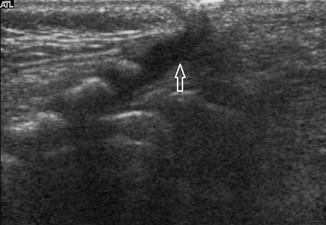

Fig. 20.7
Midline sagittal US image of the spine in a newborn shows hypoechoic splitting of the central canal in the spinal cord (arrow)

Fig. 20.8
Midline sagittal US image of the spine in a healthy newborn shows a small cystic structure just below the conus medullaris, which represents a filar cyst (arrow)

Fig. 20.9
Newborn who presented with a dimple in the gluteal crease. Sagittal US image taken at the level of the coccyx shows a hypoechoic cord-like structure extending from coccyx towards the base of the skin dimple, which represents a pseudosinus tract (arrow)
20.3 Pitfalls in Pediatric Neck Imaging
Neck masses are a common finding in the pediatric age group and are frequently referred to the radiology department for further evaluation. These lesions may have congenital, vascular/lymphatic, inflammatory, or neoplastic origins (Turkington et al. 2005). Imaging is helpful in formulating a well-defined differential diagnosis, especially when interpreted in conjunction with the patient’s age, clinical history, and physical examination. Cervical lymphadenopathy is a regularly encountered clinical problem in children and the well-known association with life-threatening diseases can lead to overaggressive and costly investigations. Although none of the US imaging findings are specific, the combination of findings during US and Doppler imaging can be strongly suggestive for a certain diagnosis. We would also like to highlight two entities, the ectopic thymus and fibromatosis colli, which should not be mistaken for worrisome pathology during the initial evaluation on US.
20.3.1 Cervical Lymphadenopathy
In most cases, the lymph node enlargement is due to benign self-limited diseases, such as viral infections causing reactive hyperplasia. Lymph node enlargement can also be due to a response to an infection in the node itself, as in lymphadenitis. Less common causes of enlargement are due to neoplastic causes such as lymphoma or metabolic causes such as Gaucher disease. There are no characteristic US and color Doppler findings that enable a definitive diagnosis in most cases, but the constellation of findings can be strongly suggestive of the underlying disease. Reactive lymph nodes are typically oval-shaped with preservation of its intra-nodal architecture and clearly defined margins. The sizes of these reactive lymph nodes are usually considerably smaller, compared to the other potential diagnoses. Infectious mononucleotic lymphadenopathy reveals similar imaging features as compared to reactive hyperplasia, but the nodes are usually larger in size and more rounded in appearance and show hypervascularity on the color Doppler US images. The enlarged nodes in lymphoma are round and hypoechoic in appearance with absent or a slit-like hilum. Lymphadenopathy caused by bacterial pathogens, cat scratch, or tuberculosis commonly presents with unilateral, heterogeneous nodal masses with indistinct margins and inflammatory changes in the adjacent fat planes. Central necrosis is commonly seen (Papakonstantinou et al. 2001) (Fig. 20.10).
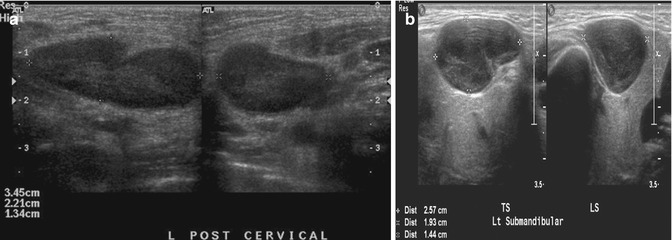

Fig. 20.10
(a) Longitudinal (left) and transverse (right) US images show an enlarged cervical lymph node due to cervical lymphadenitis in a 12-year-old girl. The lymph node still maintains its normal ovoid configuration but is enlarged. (b) Transverse (left) and longitudinal (right) US images of an enlarged left submandibular lymph node in an 11-year-old boy show the lymph node to be more rounded than usual. Histological diagnosis was Hodgkin lymphoma
20.3.2 Ectopic Thymus
In most children, an ectopic thymus presents as an asymptomatic lateral neck mass and can be easily mistaken for pathology (Nasseri and Eftekhari 2010). It is most frequently located along the pathway of thymic descent from the angle of mandible to the superior mediastinum, but it may be found in the vicinity of the superior vena cava, brachiocephalic vessels, and aorta. US is the most direct and practical imaging modality for initial investigation. The US appearance is similar to that of a normal-positioned thymus. The normal and ectopic thymuses are homogeneous, with multiple echogenic foci and echogenic septa (Fig. 20.11). An additional typical finding is that both the normal and ectopic thymus are very pliable and do not cause any compression or displacement of the adjacent structures. If the ectopic thymus is located adjacent to an artery, the ectopic thymus will conduct the arterial pulsatile motions. MRI will show a homogeneous mass with signal intensity greater than muscle on T1-weighted images and signal close to fat on T2-weighted images.
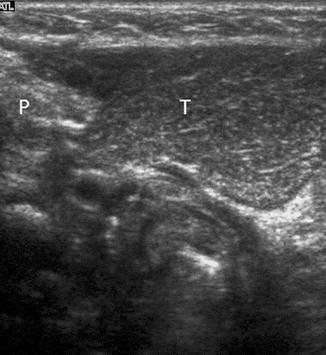

Fig. 20.11
One-year-old child who presented with a right-sided neck lump. Parasagittal US image shows a well-defined soft tissue lesion (T) with internal echogenic septa and foci, situated inferior to the right parotid gland (P). The lesion was removed and histopathologically diagnosed to be thymic tissue. CT had shown that the thymus was situated in its normal location in the anterior mediastinum (not shown). On retrospect, the multiple echogenic foci and septa seen within this lesion was consistent with thymic tissue
20.3.3 Fibromatosis Colli
Fibromatosis colli (sternocleidomastoid tumor of infancy) is a benign fusiform lesion arising from the sternocleidomastoid muscle in neonates and young infants, which causes torticollis in 14–20 % of cases. It is unilateral in most cases and has a mild predilection for the right side. It occurs in otherwise-healthy infants, but often, there is a history of birth trauma, difficult delivery, or breech presentation. Pressure necrosis or venous congestion resulting in fibroma is suggested as the underlying etiology. Clinical features and US imaging findings are usually characteristic. The non-tender, palpable neck mass generally arises within 2 weeks after birth and resolves with conservative treatment within 4–8 months. On US images, there is a fusiform enlargement of the lower two-thirds of the sternocleidomastoid muscle, which moves synchronously with the muscle during contraction (Fig. 20.12). In 90 % of cases, there is a hypoechoic rim that is likely to represent remnant surrounding normal muscle tissue. The key image is a longitudinal view of the muscle where disruption of the parallel muscle fibers by the fibroma is well appreciated. On color Doppler US imaging, this lesion can show increased vascularity. Findings which are not characteristic for this entity include extension beyond the muscle and associated lymphadenopathy. Normally, additional MRI is not necessary as the combination of clinical findings and US imaging features are characteristic (Ablin et al. 1998).
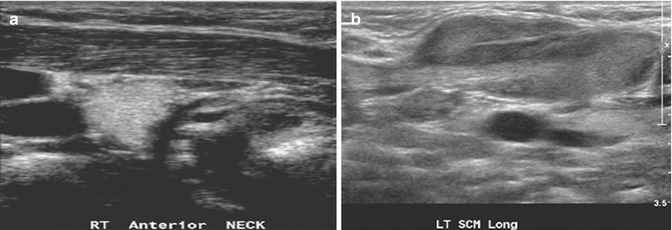

Fig. 20.12
(a) Longitudinal US image of a 6-week-old child shows a normal right sternocleidomastoid muscle. (b) Longitudinal US image of the left sternocleidomastoid muscle shows enlargement in its mid- to lower portion due to fibromatosis colli
20.4 Pitfalls in Pediatric Chest Imaging
20.4.1 Chest Radiographs
The chest radiograph is the most common investigation performed in a child. It is therefore essential to know the common normal variants and to distinguish these from pathology. In some cases, it may be necessary to use CT, US imaging or MRI to distinguish among these cases. Pitfalls in chest imaging can be technically related, anatomically related or diagnostic-related. In young infants, a chest radiograph should be obtained in the anteroposterior projection in inspiration, with the patient in a supine position. In toddlers, a standing posteroanterior view should be obtained. A radiograph taken in expiration and in the supine position may cause the heart to appear enlarged and the lungs to appear infiltrated. Expiratory films can sometimes cause lateral buckling of the trachea on the side opposite the aorta, which is a normal finding (Fig. 20.13). Proper positioning of the patient and the X-ray tube is important. The chest radiograph of a patient in a lordotic position can produce horizontal position of the posterior ribs. Cranial angulation of the tube can cause a similar effect (Fig. 20.14). In addition, rotation to one side can cause the side to which the patient is turned to be more hyperlucent than the opposite side.
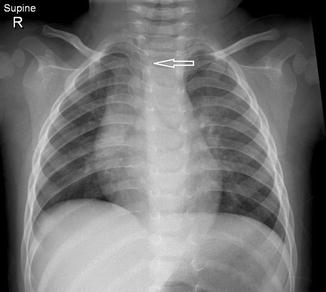
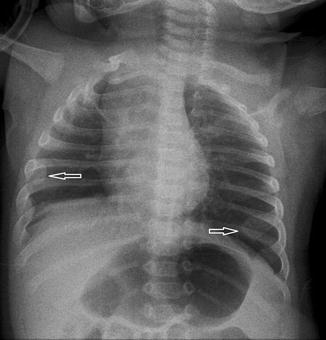

Fig. 20.13
Six-month-old infant with the chest radiograph taken with the patient slightly rotated to the right side. There is slight lateral buckling of the trachea to the right (arrow). This is a normal finding

Fig. 20.14
Chest radiograph, taken with the tube angled cranially and with the patient rotated, shows the clavicles projected asymmetrically above the chest and the ribs are horizontally oriented. Note the anterior “cupping” of the ribs which are frequently seen in neonatal chest radiographs (arrows). These are normal findings and should not be interpreted as rickets
Pleural effusion and pneumothorax may be difficult to detect in the supine position. A horizontal X-ray beam projection may aid in demonstrating a small effusion or pneumothorax. Redundant skin folds and overlying foreign objects, especially in premature infants, may mimic a pneumothorax. It is therefore important to trace the lung markings beyond the apparent fold to confirm the presence or absence of a pneumothorax. Pneumothoraces are seen adjacent to the mediastinum with the patient supine because this is the least dependent part of the chest. A pneumomediastinum is also seen in this position, and it is important to distinguish between a pneumothorax and pneumomediastinum because a pneumothorax may need to be relieved surgically, while a pneumomediastinum is treated conservatively. The pneumomediastinum will elevate the thymus, but a pneumothorax will not do so (Fig. 20.15). Overlying external structures such as hair braids, skin lesions, nipple shadows, and overlying foreign bodies are easily recognizable in most cases. In some cases oblique or lateral projections may be necessary.
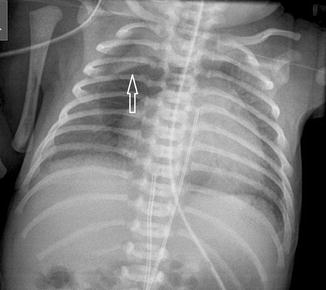

Fig. 20.15
Chest radiograph of a premature neonate shows a central lucency consistent with a pneumomediastinum. The thymus is elevated (arrow) due to the pneumomediastinum
20.4.2 Chest Ultrasound Imaging
US has been used for decades in imaging the chest. Acoustic windows through the diaphragm, thymus, suprasternal notch, subxiphoid, intercostal region, and intersegmental cartilage through the sternum may be used to evaluate the structures within the chest (Manson and Daneman 2001). US is superior to CT in the evaluation of the contents of the pleural fluid such as fibrin strands and in determining if the fluid is loculated or clear (Kurian et al. 2009) (Fig. 20.16). There are, however, pitfalls in chest US which may occur as a result of the US beam coming in contact with a highly reflected surface such as the pleura, diaphragm, lung, and liver. Mirror-image artifacts of the lung-liver interface may cause confusion when evaluating the right upper quadrant of the abdomen. Duplication of the liver, diaphragm, and lesions within the liver can cause the appearance of lesions within the lung parenchyma. Similar findings may be seen in the neck where the trachea interfaces with adjacent soft tissue which may result in duplication artifacts of the thyroid and strap muscles. In the mediastinum, the lung-mediastinum interface may similarly cause duplication of the vessels and other mediastinal structures. The pleura comprises of the parietal and visceral layers. These layers are separated by a hypoechoic potential space which can be misinterpreted as a small pleural effusion.
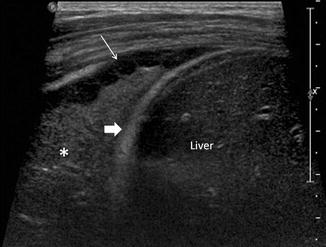

Fig. 20.16
Chest ultrasound image of a patient with pneumococcal pneumonia shows a right-sided loculated effusion with septations (thin arrow), consolidation in the lower lobe of the right lung , and the right hemidiaphragm (thick arrow). The liver is labeled
20.4.3 Chest CT
There are artifacts related to the technical aspect of performing CT, including the slice thickness, window width, tube current, voltage, and reconstruction. CT of the chest should be obtained with the chest in inspiration. However, young children may not be able to hold their breath; therefore, CT studies may be acquired in the expiratory phase. This causes decreased aeration in the lungs, giving rise to the appearance of ground-glass attenuation which may mimic pulmonary disease (Fig. 20.17). Although it has been recommended to perform scans in both lateral decubitus positions and in expiration to differentiate pulmonary disease or ground-glass change, this is often not practiced, due to awareness of the increased radiation dosage and to avoid unnecessary radiation to the patient. Proper windowing should be performed to differentiate pulmonary nodules from pseudonodules (Goya et al. 2009). Pseudonodules may be due to normal anatomical structures such as the costal cartilages and ribs, sternum, vascular structures, or diaphragm. Interpretation of the images in all three orthogonal planes is helpful and should resolve most such cases of pseudonodules. A common example is a small pulmonary vessel seen on a single axial image, interpreted as a small nodule. When seen on another plane, a vessel should appear linear and branching. Another common pitfall is basal-dependent atelectasis wrongly interpreted as being a lung nodule. These typically occur in patients lying in the supine position.
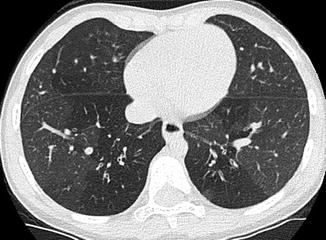

Fig. 20.17
Axial CT image of a child taken in expiration shows a mosaic pattern. Areas of ground-glass opacification are seen interspersed between areas of normally inspired lung
20.4.4 Anatomic Pitfalls
20.4.4.1 Thymus
The thymus gland is very commonly mistaken for pathology. It can mimic a cardiac or mediastinal mass, if very large. Features that suggest that the mass is a normal thymus gland include its triangular shape, with a notch seen at its junction with the heart, and its wavy or straight lateral borders (Goya et al. 2009). A chest radiograph taken with the patient slightly rotated to the right can result in the thymus being projected over the right upper lobe and be mistaken for pneumonia or mediastinal mass (Fig. 20.18). In doubtful cases, US examination may be used to distinguish the thymus from a pathological mediastinal mass. On US images, the thymus has a homogeneous echogenicity with some echogenic septa and foci within it (Fig. 20.19). It also does not displace or compress adjacent vascular structures, unlike a mediastinal mass.
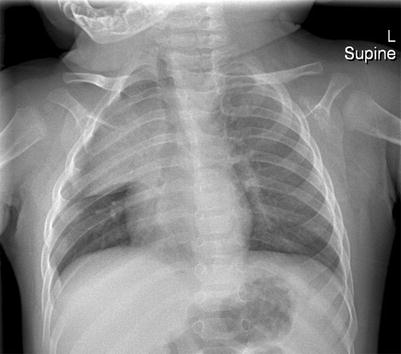
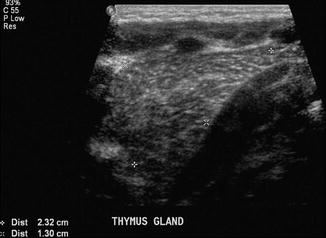

Fig. 20.18
Two-month-old infant who was rotated slightly to the right when the chest radiograph was taken. The thymus gland is prominent and projected over the right upper hemithorax

Fig. 20.19
US image of a normal thymus gland which appears as a soft tissue mass with some echogenic septa and foci within it. It does not displace or compress adjacent vascular structures, unlike a mediastinal mass
20.4.4.2 Mediastinum
The “ductus bump” is usually seen in newborn infants and represents the dilated ductal infundibulum of the closing ductus arteriosum (Fig. 20.20). It is seen in the region of the aortic knob and is usually not seen beyond the neonatal period. Another normal finding one should look out for is calcification of the ligamentum arteriosum, usually located between the aortic knob and pulmonary artery (Fig. 20.21). It should not be confused with a foreign body.
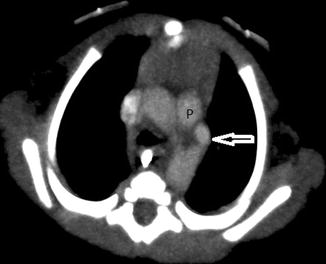
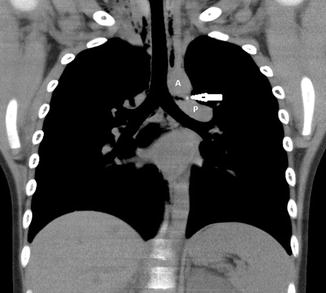

Fig. 20.20
Newborn presenting with respiratory distress. Contrast-enhanced axial CT image shows a rounded density (arrow) adjacent to the pulmonary artery (P) consistent with an aneurysm of the ductus arteriosum. This is a normal structure in the neonatal period and disappears soon after

Fig. 20.21
Twelve-year-old girl who presented with chest pain and pneumomediastinum. Coronal CT image performed to look for a foreign body shows pneumomediastinum and a speck of calcification (arrow) located between the aortic arch (A) and pulmonary artery (P), typical of calcification of the ligamentum arteriosum
20.4.5 Diagnosis-Related Pitfalls
20.4.5.1 Round Pneumonia
Round pneumonias are spherical or ovoid pneumonias commonly seen in children. They may mimic a lung or mediastinal mass (Fig. 20.22). Recognition of this entity may prevent further unnecessary imaging and intervention. Careful evaluation of the density may reveal air bronchograms within the density or in the surrounding lung parenchyma. Close clinical correlation is also beneficial as these patients present with an abrupt onset of fever and cough and possess findings on auscultation that suggest pneumonia. Round pneumonias resolve on follow-up radiographs after treatment.
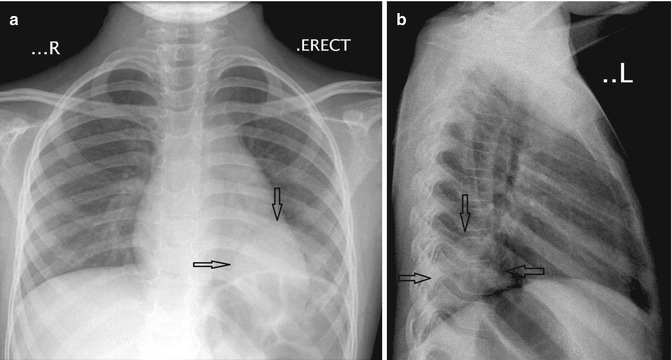

Fig. 20.22
Six-year-old child who presented with cough and fever. (a) Anteroposterior and (b) lateral chest radiographs show a rounded density located just above the left hemidiaphragm, consistent with a round pneumonia (arrows)
20.4.5.2 Paravertebral Mass Versus Bronchopulmonary Sequestration
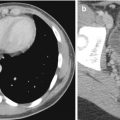
Pulmonary sequestrations are embryonic masses of lung tissue that do not communicate with the bronchial tree and receive their blood supply from anomalous systemic arteries. They are often located close to or in contact with the paravertebral region, and it may be difficult to differentiate these lesions from neuroblastomas which may also be situated in this region. Neuroblastomas also have a systemic arterial supply which may be demonstrable on color Doppler US studies (Manson and Daneman 2001). Neuroblastomas contain calcifications which are seen in more than 90 % of cases on CT. Differentiation between these two entities may be difficult if the lesion does not contain calcifications. In these cases, it may be necessary to perform 24-h urine vanillylmandelic acid (VMA) studies or biopsy to exclude neuroblastoma (Fig. 20.23).
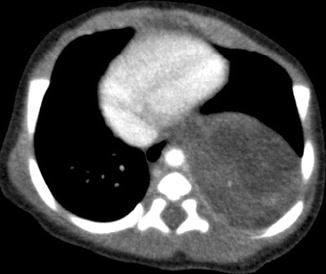

Fig. 20.23
Two-day old neonate who presented with a large paravertebral mass that was detected antenatally. Axial contrast-enhanced CT image shows a large soft tissue lesion in the paravertebral region that was later resected and found to be a bronchopulmonary sequestration
20.5 Pitfalls in Pediatric Abdominal Imaging
The evaluation of a child with abdominal pain is the commonest indication for imaging the abdomen. There are many causes of abdominal pain and one of the most frequent indications is to exclude acute appendicitis. Other indications for imaging the abdomen are to diagnose intestinal malrotation with/without volvulus and necrotizing enteritis. Patients with complications arising from a Meckel diverticulum may also be frequently overlooked. There are many imaging modalities that can be used in the evaluation of these patients including abdominal radiographs, US imaging, CT, MRI, and fluoroscopic-guided contrast examinations. There are pitfalls related to all these modalities.
20.5.1 Appendicitis
20.5.1.1 Radiographic Pitfalls
Children with appendicitis usually present with periumbilical or right iliac fossa (RIF) pain. An oval and lamellated appendicolith seen on the abdominal radiograph in a patient with RIF pain is diagnostic of appendicitis in more than 90 % of cases. However, this finding is present in only a minority of cases. Other features are nonspecific, such as ileus or paucity of right lower quadrant bowel gas. Thus, the radiographic findings are often unhelpful and the diagnosis of appendicitis should not be excluded on the basis of a normal abdominal radiograph.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree