Jean Noel Buy1 and Michel Ghossain2
(1)
Service Radiologie, Hopital Hotel-Dieu, Paris, France
(2)
Department of Radiology, Hotel Dieu de France, Beirut, Lebanon
33.2 General Features
33.2.1 Definition
33.2.2 Epidemiology
33.2.3 Risk Factors []
33.2.5 Methods of Verification
33.3.1 Endometritis
33.3.2 Tubal Lesions
33.3.3 Ovarian Involvement
33.3.4 Peritonitis
33.3.5 Vascular Thrombosis
33.3.6 Lomboaortic Lymphadenopathy
33.4.2 Hydrosalpinx
33.4.3 Adhesions
33.4.4 The Two Main Complications
Abstract
See Chap. 20
33.1 Anatomy and Histology of the Tube
Pelvic inflammatory disease (PID) refers to a spectrum of inflammation involving the female upper genital tract (UGT) and the supporting tissues secondary to an infection. PID may be confined to the uterus (endometritis), to the fallopian tubes (salpingitis), the ovaries (oophoritis), both realizing a tubo-ovarian abscess, the supporting ligaments (parametritis), or may involve several of the above uterine appendages. In severe cases, it may be complicated with peritonitis.
33.2 General Features
33.2.1 Definition
Pelvic inflammatory disease (PID) refers to a spectrum of inflammation involving the female upper genital tract (UGT) and the supporting tissues secondary to an infection. PID may be confined to the uterus (endometritis), to the fallopian tubes (salpingitis), the ovaries (oophoritis), both realizing a tubo-ovarian abscess, the supporting ligaments (parametritis), or may involve several of the above uterine appendages. In severe cases, it may be complicated with peritonitis.
33.2.2 Epidemiology
Although the cervix acts as a barrier to the entry of microorganisms into the endometrial cavity, most types of endometritis result from ascending infection. During menstruation, abortion, parturition, and instrumentation (curettage, insertion of uterine device, cervical conization), the cervical barrier to infection is breached, allowing normal vaginal flora access to the endometrial cavity, but colonization and infection are uncommon.
Endometritis related to bacteremia or secondary spread to the uterus from a primary salpingitis (tuberculosis) is unusual.
Rarely, an infection of the fallopian tubes is caused by direct spread from nearby pelvic organs, most often the appendix (Fig. 16.4).
33.2.3 Risk Factors [3]
Young woman (mean 25 ± 6).
Nulliparous, 58%.
Oral contraceptive, 34%.
IUD, 8%, or use of an IUD in the past, 30%.
Mean age of the first coitus, 17.
Previous history of sexual transmitted disease, 12%.
Previous history of PID, 30%.
A change of the sexual partner in the preceding 2 months in 24% of patients.
33.2.4 Clinical and Biological Findings
The diagnosis of PID is usually made clinically. Clinical characteristics are reported in Table 33.1.
Table 33.1
Clinical findings in PID
Common |
Lower abdominal pain usually bilateral (>90%) |
Adnexal tenderness or palpable adnexal mass |
Pain on manipulation of the cervix |
Purulent cervicitis |
Not always present |
Fever, chills |
Abnormal vaginal discharge, abnormal uterine bleeding |
Dysuria, dyspareunia |
Nausea, vomiting, tender and rigid abdomen (in case of peritonitis) |
Biological findings are not always present. They are reported in Table 33.2.
Table 33.2
Biological findings in PID
Leukocytosis |
Elevated erythrocyte sedimentation rate |
Elevated C-reactive protein |
Elevated CA 125 |
However, clinical diagnosis of PID has severe limitations.
Infections that are asymptomatic or have atypical presentations also occur [1]. Many women with chronic sequelae of PID, such as infertility, have no known history of the disease.
On the other hand, only two-thirds of women with clinical diagnosis of PID had PID at laparoscopy. Some patients have a normal laparoscopy, while others have another explanation for their symptoms such as acute appendicitis, pelvic endometriosis, and hematoma of a corpus luteum cyst or ectopic pregnancy.
33.2.5 Methods of Verification
1.
Laparoscopy
Laparoscopy is considered the method of choice, but it necessitates anesthesia and may lead to complications.
PID may be diagnosed on the visual signs of commonly accepted findings [1, 2]:
Acute salpingitis is diagnosed when a hyperemic edematous fallopian tube with no sign of intraluminal pus is seen.
A pyosalpinx is diagnosed when an enlarged edematous tube with a partial or total destruction of the fimbriated end is seen. In order to confirm the diagnosis, the tube is incised and pus is drained.
A tubo-ovarian abscess is diagnosed when the ovary and the tube cannot be distinguished from each other, forming an adnexal complex mass with abscess formation.
Presence of purulent material in the pelvis.
2.
Endometrial biopsy is a simple office procedure that can be performed without anesthesia. A biopsy showing plasma cell endometritis has been found to have a sensitivity of 89% and specificity of 67% for the diagnosis of PID [2].
3.
Microbiological tests of UGT infection (endometrium, pelvis, or fallopian tube).
The main infectious agents are:
Chlamydia, mycoplasma
Anaerobic gram-negative rods
Mycobacterium tuberculosis
Fungal agents
Viral agents: HSV, CMV, HIV
4.
Patients are considered to have UGT infection if their laparoscopic, histologic, or microbiologic tests are positive.
33.3 Acute Manifestations of PID
33.3.1 Endometritis
33.3.1.1 Definition
Normal Endometrium
B lymphocytes are mainly present in aggregates in the basalis.
T lymphocytes are relatively uncommon in the proliferative phase but increase during the secretory and menstrual phases.
Polymorphonuclear leukocytes (PMNs) are present in small numbers throughout the cycle but do not become evident in large numbers before the tissue breakdown and necrosis is associated with menses.
Plasma cells are not normally present in the endometrium.
Acute Endometritis
Polymorphonuclear leukocytes (PMNs) of moderate to large numbers on nonbleeding endometrium, aggregates of PMNs in the stroma, microabscesses, or filling and disruption of glands by PMNs.
Chronic Endometritis
Identification of plasma cells (cells with eccentric nuclei, with a pale perinuclear halo related to the Golgi apparatus), but lymphocytes, macrophages, and rare PMNs may be present.
33.3.1.2 Imaging Findings
EVS
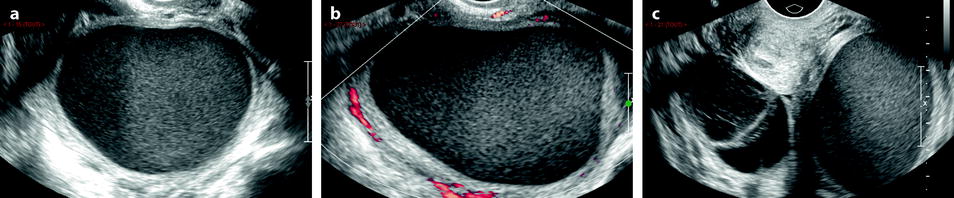
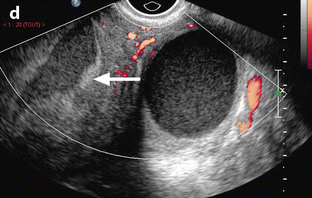
Fig. 33.1
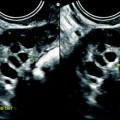
Left tubo-ovarian abscess with pyometria. Thirty-nine-year-old woman. IUD and pelvic pain with leucorrhea for 2 weeks; US is performed after the IUD has been removed. (a, b) Longitudinal: EVS (a) depicts a left cystic mass with a ground-glass pattern suggestive of an endometrioma; on color Doppler (b), few vessels are visualized in the wall. (c) On transverse view the left ovarian cystic mass is displayed; a functional hemorrhagic cyst with a fishnet pattern is seen in the right ovary. (d) Section through the uterus and the left mass displays pus in the endometrial cavity (arrow). Preoperative diagnosis: as far as a groundglass pattern can also be seen in an ovarian abscess associatiation with the presence of pus in the endometrial cavity suggested the diagnosis of ovarian abscess. At surgery, an abscess of the left ovary was drained
MR
Thickened heterogeneous endometrium.
Fluid in the endometrial cavity.
Enhancing endometrium.
CT
Gas when present is better displayed than on US or MR.
This endometritis can be associated with myometritis (Fig. 33.2).
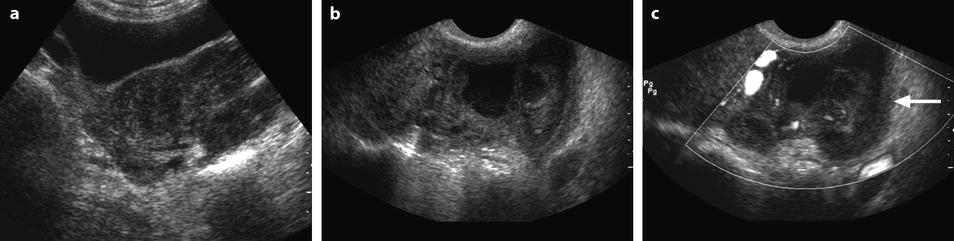
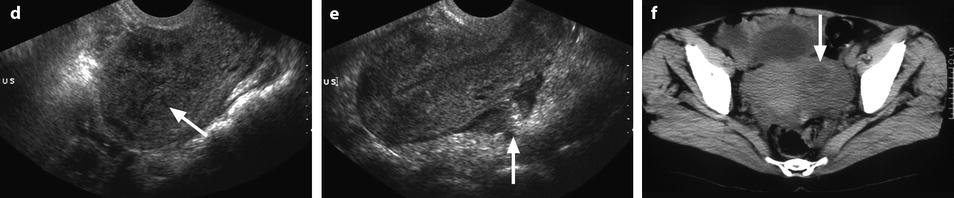
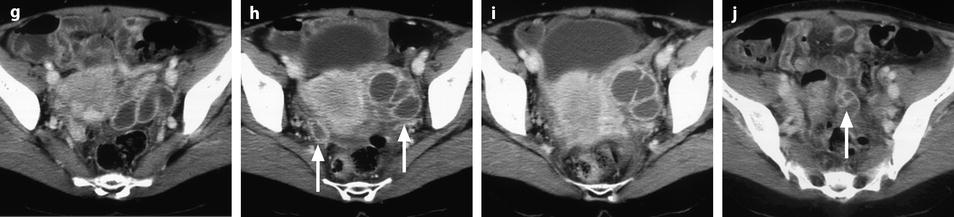
Fig. 33.2
Bilateral pyosalpinx complicating chronic salpingitis and myometritis with pelvic peritonitis. A 49-year-old woman with sudden left pelvic pain and increased white cell count. (a) TAS (transverse view) displays bilateral echogenic adnexal masses. On the right, the elongated shape is very suggestive of a tubal origin. (b, c) EVS (b) with color Doppler (c), sagittal view of the left adnexal mass. The left adnexa has a mixed echogenic structure; the wall is thickened and hypervascularized (arrow); the outer wall has indistinct margins. (d) EVS, sagittal view of the uterus: Hypoechogenic areas in the myometrium (arrow); their precise signification was not clearly established; however, the uterus was soft and inflammatory at operation very likely related to a myometritis. (e) EVS, sagittal view: echogenic material in the cul-de-sac is related to peritonitis (arrow). (f) CT scan without contrast: left adnexal mass containing a hyperdense rim very suggestive of an abscess (arrow). (g–j) CT scan after IV contrast: bilateral pyosalpinx with contrast uptake in the wall of the fallopian tubes (arrows in h). Peritoneal fluid, debris in the cul-de-sac, and high contrast uptake in the bowel walls (arrow in j) are suggestive of peritonitis, which was confirmed at surgery and pathology
33.3.2 Tubal Lesions
33.3.2.1 Definition
Acute salpingitis: a purulent inflammatory process usually secondary to the passage of bacteria from the uterine cavity into the tubal lumen.
Pyosalpinx: if the tube becomes occluded, the purulent exudate fills and distends the lumen [1].
33.3.2.2 Pathological Findings
1.
The bacteria penetrate the cells and the intercellular junctions with cell lysis and sloughing.
2.
They penetrate the subepithelial connective tissue. A brisk diapedesis of granulocytes occurs from capillaries into the mucosa and lumen, and there is vascular engorgement and edema of all tubal layers.
3.
In severe cases, transudation of plasma proteins results in a fibrinous exudate on the serosal surface, which reddens because of vascular dilatation.
4.
As the lumen fills with granulocytes and cellular debris, and the tube distends, pus may be seen dripping from the fimbriated end in patients undergoing laparoscopy.
33.3.2.3 Imaging Findings
The main imaging findings are reported in Table 33.3.
Table 33.3
Imaging findings in PID
Salpingitis |
1. Morphologic findings |
Tubal shaped thickened structure |
2. Pattern |
US: homogeneous echogenic, sometimes mucosa hyperechoic, muscularis hypoechoic |
CT: tissular density |
MR: low signal on T1, intermediate signal on T2, possible double layered wall |
3. Vascular findings |
Hypervascularity |
Pyosalpinx |
1. Morphologic findings |
Tubal shape cystic structure, plicae, thickened wall |
2. Pattern of cystic content |
US: echoic with fluid debris |
CT: density superior to urine |
MR: intermediate signal on T1, high on T2 |
3. Vascular findings |
Color Doppler: regular arterial vessels |
DCT and DMR: |
– At the arterial phase: regular arterial vessels |
– On the delayed phase: marked accumulation and diffusion of contrast in the wall |
33.3.2.4 Ultrasound Findings
The sensitivity in detection of tubal abnormality in patients with laparoscopic PID is very variable from low to as high as 93% in Patten’s series [4]. Anyway an endovaginal US consistent with PID may be helpful in avoiding laparoscopy.
1.
Acute Salpingitis
At this stage, US can be normal or show only nonspecific anechoic or echoic fluid in the cul-de-sac [5].
When salpingitis is seen, patients show an indistinct elongated tubular shape, echogenic, and rather homogeneous mass, in close proximity, but separated from the ovary. In the central part, hyperechoic mucosal lining can be displayed [6]. The wall is thickened and hypoechoic.
This aspect can be very difficult to distinguish from appendicitis; however, in this last case, a well-defined central hyperechoic mucosal layer [7–9] is usually seen.
2.
Pyosalpinx (Fig. 33.3)
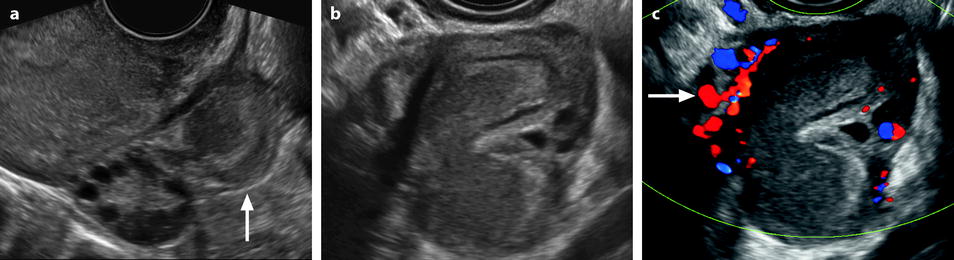
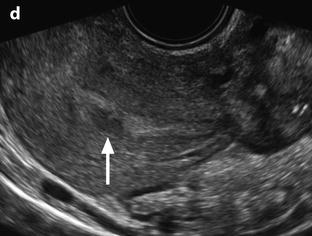


Fig. 33.3
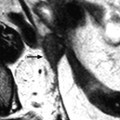
Left pyosalpinx. Nineteen-year-old woman. Pelvic pain without vaginal discharge. Sedimentation rate 90, RCP 46, and CA 125: 435 UI/ml. EVS longitudinal (a) displays a left tubular collection (arrow), in contact with the posterior border of the ovary. Transverse view EVS without (b) and with (c) color Doppler displays a left extraovarian collection with a tubular shape containing an echogenic fluid; the wall is thickened, highly vascularized (b) which is in favor of an active acute inflammatory process (arrow). Transverse view of the uterus (d) displays some echogenic fluid in the endometrial cavity (arrow). Prospective diagnosis: left pyosalpinx. Vaginal sample: chlamydiae infection. Coelioscopy confirmed the diagnosis of pyosalpinx with a peritoneal reaction
Like in a hydrosalpinx, a convoluted pear-shaped cystic mass with echogenic wall [13] separated from the ovary with incomplete septa resulting from folding of the wall of the tube is seen. On the outer wall, adhesions may be seen.
However, different findings usually are suggestive of a pyosalpinx:
Most often, it contains diffuse internal echoes or fluid-debris level [5].
The protrusion of the mucosa into the lumen causing the so-called cogwheel sign [14, 15] is highly suggestive of an acute inflammatory process [10].
The wall is markedly thickened (>5 mm).
On color Doppler, blood vessels are visualized in the wall and the septa (Figs. 33.3 and 33.4).
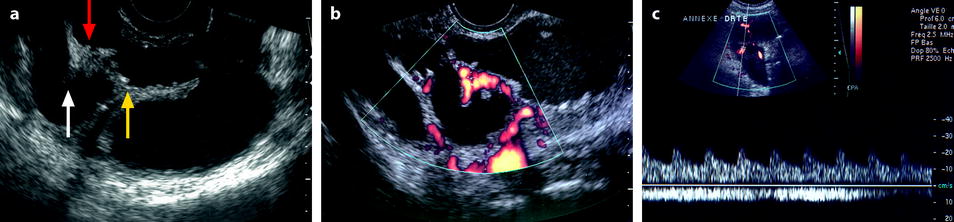
Fig. 33.4
Right pyosalpinx. EVS (a) displays a tubal collection with echogenic content. Thickened plicae are elongated (white arrow) while others resemble papillary projections (red arrow). Some echogenic foci are visualized on the surface of the plicae (yellow arrow). Color Doppler (b) displays a hypervascularization in the wall and in the plicae. Pulse Doppler (c) depicts in the arterial vessel a low-resistive index
Conversely, a beads-on-a-string sign, a marker of chronic persistent wall damage, is not seen in acute PID, while it is present in 80% of hydrosalpinx group in Molander’s series [10].
3.
Association of salpingitis on one side and pyosalpinx can be displayed (Fig. 33.5).
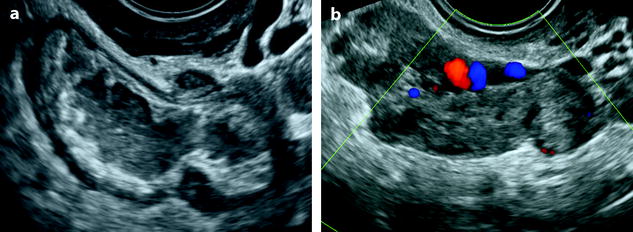

Fig. 33.5
Right pyosalpinx and left salpingitis. Thirty-five-year-old woman, lumbar pain, metrorrhagia and vaginal discharge, asthenia, fever, and CRP 50. (a) EVS of the right adnexa displays a dilated tube containing hypoechogenic fluid and internal debris, with a very thickened wall. (b) EVS of the left adnexa depicts a tube with a significant thickened wall. Dilated vessels are visualized in the wall or in the mesosalpinx. Prospective diagnosis: PID with bilateral tubal involvement. Vaginal sample: chlamydiae and mycoplasma infection
33.3.2.5 CT Findings
2.
In case of a pyosalpinx, the tubes exhibit a greater degree of wall thickening and enhancement and fill with complex fluid [16] (Fig. 33.6).
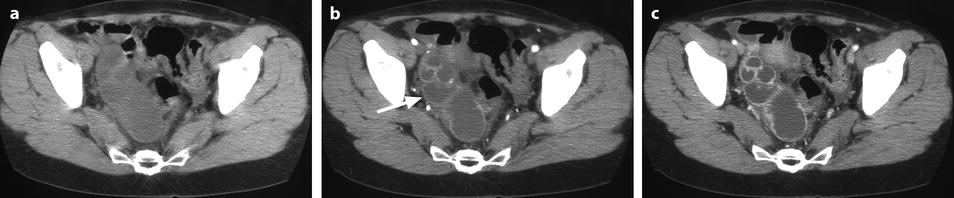
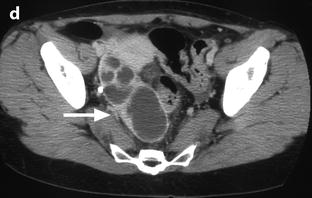


Fig. 33.6
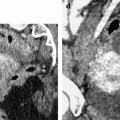
Right pyosalpinx. CT without injection (a) shows a right tubular collection with some fluid in the Douglas. At the arterial phase 20 s after injection (b), regular arterial vessels are displayed in the wall (arrow) and in the folding of the tube. 30s after injection (c), contrast uptake is visualized in the wall. On the delayed phase performed 4 min after injection (d), a characteristic contrast diffusion and contrast enhancement is depicted (arrow)
33.3.2.6 MR Findings
2.
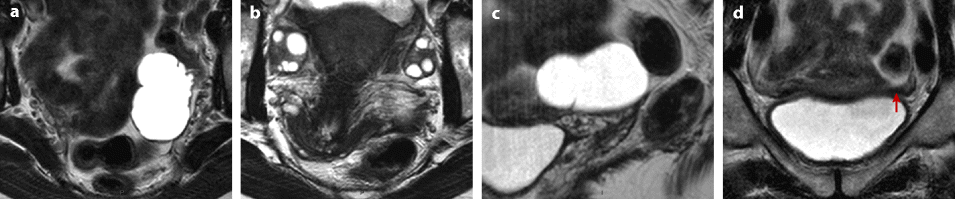
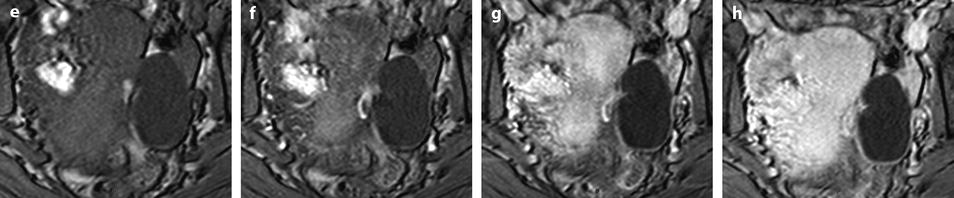
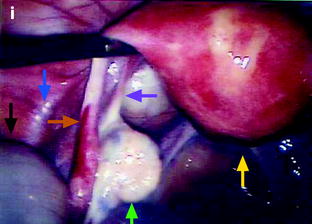
Dilatation of the fallopian tube, which may involve all the tube, or can be limited to the ampulla and the infundibulum.



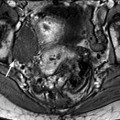
Fig. 33.7
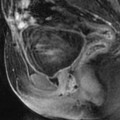
Left hydrosalpinx of the ampulla with a normal isthmic portion. Forty-four-year-old woman. Ulcerative hemorrhagic colitis. Left pelvic pain, nausea, vomiting. MR axial T2 (a) and sagittal T2 (c) display a left adnexal mass with irregular margins, a tubular shape with a slightly thickened wall (except in the anterior part). On axial T2 (b) the left ovary is normal definitely confirming the extraovarian origin of the collection and the diagnosis of hydrosalpinx. On coronal T2 (d) the proximal portion of the left tube is of normal caliber without any fluid (red arrow) Fig. 33.7 (continued) (e–g) DMR without injection (e), at the arterial phase (f), at the venous phase (g) and on the delayed phase (h) depict only contrast enhancement in the thickened portion of the wall. (i) Coelioscopy confirmed the diagnosis of hydrosalpinx of the ampulla (yellow arrow) with a normal isthmic portion (brown arrow). Uterus (black arrow), left ovary (green arrow) round ligament (blue arrow), tuboovarian ligament (purple arrow). Left salpingectomy was performed. At pathology: hydrosalpinx. This case outlines the possibility for an hydrosalpinx to be limited to the ampulla and to the infundibulum
3.
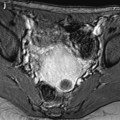
Findings suggestive of a pyosalpinx (Fig. 33.8).
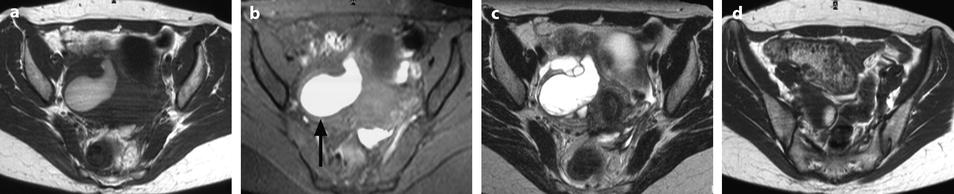
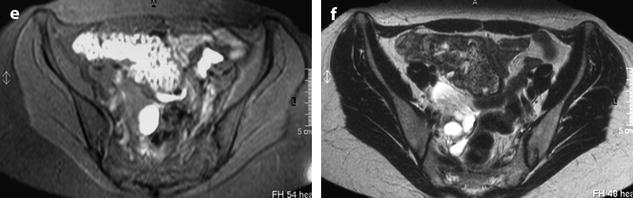


Fig. 33.8
Right pyosalpinx with high signal intensity on T1 and peritonitis. (a–c) Axial MR with T1 (a), T1 fat suppression (b), and T2W (c), images display a right tubular collection with a signal intensity of the content intermediate (between pelvic muscle and adipose tissue) on T1, high on fat suppression (arrow) and high, superior to signal intensity of urine on T2. These characteristics of signal are typical for a pyosalpinx. (d–f) Axial T1 (d) fat suppresion (e) and T2 (f) display a thickening of the mesosalpinx better displayed in f. The mesosalpinx was markedly congestive at surgery. At pathology the tube was edematous and covered with false membranes with fibrino leukocyte deposits related to peritonitis
The wall is usually thickened.
Signal intensity of the content is typically intermediate on T1 (between signal intensity of pelvic muscle and adipose tissue), high on fat suppression, and increases on T2 with a signal close to urine.
These charcteristics are quite different from those of endometriosis where signal intensity is typically close to adipose tissue on T1, high on fat suppression while signal intensity typically decreases on T2 (intermediate between pelvic muscle and adipose tissue) [17].
33.3.3 Ovarian Involvement
33.3.3.1 Tubo-Ovarian Abscess (TOA)
Ovarian involvement by PID is almost always secondary to salpingitis and typically takes the form of a tubo-ovarian abscess [18]. Inadequately treated PID may lead to unilateral or bilateral TOA [4]. Unfortunately, clinical symptoms secondary to uncomplicated salpingitis and TOA are often similar and up to 20% of patients with TOA lack clinical signs of infection [19].
Macroscopic and Radiologic Findings
They are summarized in Table 33.4.
Table 33.4
Macroscopic and radiologic findings of TOA
– Uni- or bilateral |
– Tubular or round shape |
– Most commonly cystic |
– Uni- or multilocular or multicystic |
– 7–12 cm |
– Thickened wall, irregular margins |
– Complete or incomplete septa |
– Rarely, mostly solid |
Vascular Findings
Two types of findings: angiogenesis and increase of the vascular permeability are responsible for characteristic findings on DCT or DMR.
US Findings
Morphologic Findings
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree