
Percutaneous Intervention of Malfunctioning Surgical Portosystemic Shunts
Portosystemic shunts are designed to decompress the portal venous system in patients with significant portal hypertension. Portosystemic shunts can be broadly categorized into the following two groups: (1) surgically created extrahepatic shunts and (2) percutaneous transjugular intrahepatic shunts (see Section 1, Chapters 4–6).
Surgical portosystemic shunting is performed primarily in patients with hepatic cirrhosis or the Budd-Chiari syndrome. Despite the fact that the disease entities can be quite different, both groups of patients can present with or develop clinical signs and symptoms of portal hypertension. Variceal hemorrhage and progressive liver failure are the most devastating complications of either of these two diseases. Portosystemic shunting may be performed to address these complications or as a primary treatment modality. A variety of surgical procedures have been described to decompress the congested liver and portal venous system, including creation of a mesoatrial, mesocaval, portocaval, inferior vena cava-atrial, portoatrial, portopulmonary, splenorenal, and splenopulmonary shunts.1–8
Despite the fact that these shunts are constructed of relatively large-diameter (12–22 mm) Dacron or polytetrafluoroethylene (ePTFE, W. L. Gore and Associates, Flagstaff, AZ) grafts with (8–12 mm) end-to-side or side-to-side anastomoses, stricture formation at the surgical anastomosis is reported in 5 to 44% of patients.1,9–11 Shunt thrombosis is estimated to occur in 5 to 15% of patients11,12 but is probably higher because many patients may not actually hemorrhage following shunt occlusion.
This section addresses the radiographic imaging of and percutaneous techniques available for salvage of malfunctioning surgically created portosystemic shunts.
 Noninvasive Imaging
Noninvasive Imaging
As progressive deterioration of shunt function occurs as a result of an anastomotic stenosis or thrombus formation, blood flow through the shunt decreases and portal venous pressure concomitantly increases. Patients typically develop weight gain and increasing abdominal girth secondary to the accumulation of ascites and hepato (spleno)megaly. Most patients are acutely aware of such changes, even when subtle.13 Some patients also may experience pain from congestive hepatomegaly, and a few (4%) unfortunate patients will present acutely with variceal hemorrhage.11,13–19
Although the diagnosis of shunt malfunction is usually self-apparent, noninvasive imaging studies typically are performed for confirmation of shunt obstruction or occlusion. Magnetic resonance imaging (MRI), ultrasound, and computed tomography (CT) all have been used for evaluation of shunt malfunction, and each modality has advantages and disadvantages.
Magnetic Resonance Imaging
Numerous studies have demonstrated that MRI offers an accurate means of evaluating portosystemic shunts in its ability to detect blood flow and provide multiplanar imaging.20–22 Using spin-echo techniques, flowing blood produces no signal. Patent vessels or grafts thus appear as areas devoid of signal (Fig. 8–1). Axial, sagittal, and coronal images can be reconstructed so that the entire length of a synthetic graft can be visualized. Typical imaging parameters for spin-echo axial imaging would include a repetition time (TR) of 900 milliseconds, an echo time (TE) of 20 to 30 milliseconds, and a 10-mm slice thickness. A TE of 30 milliseconds and TR of 700 to 1260 milliseconds can be used for sagittal and coronal imaging.20,21 On T2-weighted images or with even-echo rephasing techniques, thrombus stands out as a high signal intensity area against the low signal intensity background of flowing blood. Two- or three-dimensional MRA/FLASH (fast low-angle shot) images (TR/TE/angle 20-70/8-12/15-40) and gradient refocused echo images (Fig. 8–2) also will depict flowing blood as a region of high signal intensity against the dark background of the surrounding soft tissues.22
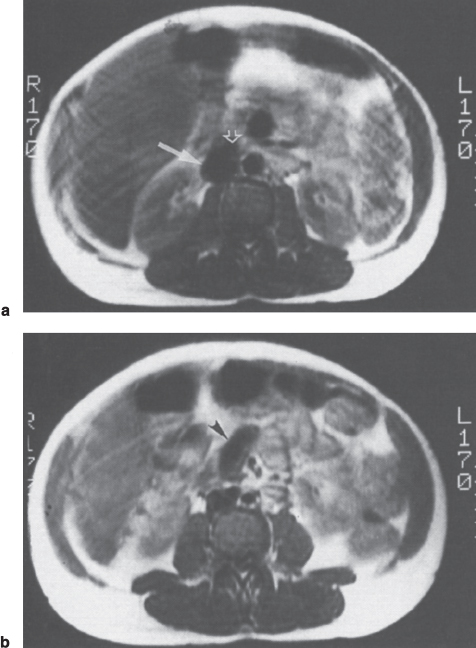
FIGURE 8–1. Spin-echo T1-weighted (TR-650 msec, TE-20 msec) magnetic resonance image in a patient with mesocaval shunt. (a) Absence of signal within the inferior vena cava (arrow) and graft (open arrow) is consistent with flow within this portion of the shunt. Note that the actual anastomisis is clearly delineated, (b) Absence of signal in the “body” of the shunt (arrowhead) is evidence of patency.
Bernardino et al20 reported that spin-echo MRI accurately assessed patency (n = 21) and thrombosis (n = 2) in 23 (82%) of 28 patients with selective and nonselective shunts. Angiography was used as the gold standard to which the magnetic resonance examinations were compared. MRI was not able to assess shunt patency in five (18%) cases in which steel embolization coils had been previously placed to obliterate gastroesophageal varices. A second study by Chezman and Bernardino21 reported that MRI correctly assessed the status of nine mesoatrial shunts, including the demonstration of four cases of shunt stenosis. Of 11 MR examinations, angiographic findings correlated with the results of MRI in all cases.
Advantages of MRI include its highly detailed multiplanar images that are obtained without contrast, ionizing radiation, or vascular catheterization. (More advanced magnetic resonance angiography flow specific sequences may require the use of intravenous gadolinium for optimization of images.) In most cases, however, angiographic evaluation of the shunt will be required for hemodynamic measurements.
Ultrasound
Both duplex Doppler and color-flow Doppler ultrasound techniques can be used for accurate noninvasive evaluation of surgical portosystemic shunts and may be particularly useful in the acutely ill patient and in those who cannot tolerate the confines of MRI. These techniques are noninvasive, readily available, and relatively inexpensive. Thus, ultrasound can be used in the follow-up of patients on a regular basis after the initial procedure.
In patients who have synthetic grafts (mesoatrial shunt; mesocaval shunt with interposition graft), the echogenic walls are readily identified by duplex Doppler imaging. Once the graft is identified and its course is mapped out, either duplex or color-flow Doppler sonography can be used to establish patency (Fig. 8–3).23 Although duplex Doppler is accurate for portocaval, mesoatrial, and mesocaval shunt evaluation,21,24–26 the complex anatomic position of the splenorenal shunt is such that duplex imaging alone is often inadequate for evaluation. Grant et al27 reported that duplex ultrasound alone could fully evaluate only 4 (29%) of 14 splenorenal shunts, whereas color-flow Doppler ultrasound could fully assess all 14 shunts.27 Color-flow Doppler ultrasound appears to have better penetrance in areas where overlying bowel gas can obscure routine duplex Doppler images. Overall, color-flow Doppler ultrasound can greatly improve the accuracy of ultrasound imaging, allowing prediction of the direction of flow and shunt patency versus thrombosis with a sensitivity and specificity as high as 100%.23
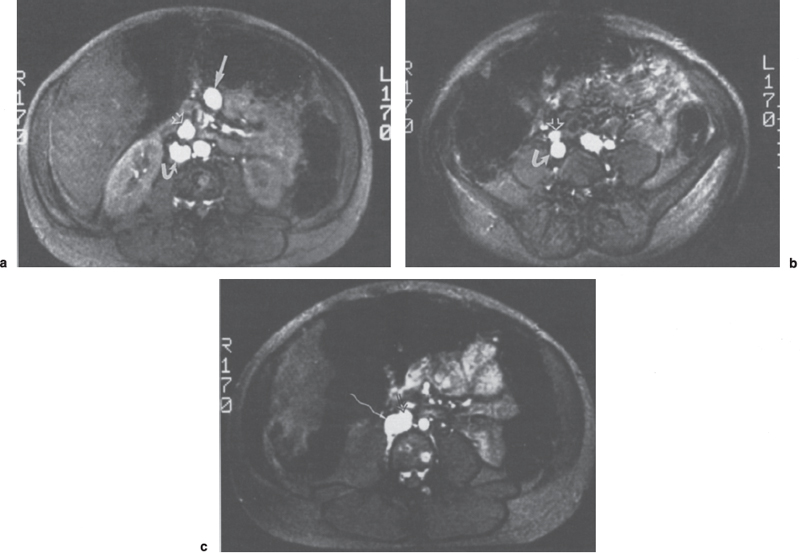
FIGURE 8–2. Gradient refocused echo (TR-22 msec, TE-13 msec) images of mesocaval shunt. (a) High signal intensity is noted within the superior mesenteric vein (arrow), mesocaval shunt (open arrow), and inferior vena cava (curved arrow) consistent with flow through all three structures. (b,c) The anastomosis of the shunt graft (open arrow) to the inferior vena cava (curved arrow) is clearly delineated.
Duplex ultrasound is capable of detecting a clot within the portal vein and portosystemic shunt following surgery. Rice et al27 reported that an intrahepatic portal vein clot was detected in 69% and 67% of patients with end-to-side shunts and distal splenorenal shunts, respectively. The significance of this clot, however, is not fully understood. The presence of a clot does not necessarily imply impending shunt thrombosis because the patency rates for these shunts far exceed the percentages of cases in which thrombus is detected. Further evaluation of the significance of this clot is warranted; however, the present data do not indicate that thrombectomy or thrombolytic therapy is required in all cases in which nonocclusive thrombus is detected. Each patient must be evaluated individually and, should pending shunt or portal vein thrombosis be suspected, angiographic and hemodynamic evaluation is warranted.
Computed Tomography
Contrast-enhanced dynamic CT may be used for shunt evaluation. In addition, information about the presence or absence of varices, ascites, hepatosplenomegaly, cirrhotic liver disease, intrahepatic masses (hepatocellular carcinoma), and pleural effusions also can be assessed.
To perform dynamic CT, a 50-mL bolus of 60% contrast material is injected followed by a continuous infusion of 100 mL of 30% contrast. Imaging is initiated following completion of the bolus injection with 10-mm contiguous axial sections obtained to include the entire length of the shunt. Fishman et al28 reported that contrast-enhanced CT accurately diagnosed two occluded and one stenotic mesoatrial shunt and confirmed patency in an additional two shunts (Figs. 8–4 and 8–5). Other authors confirmed the value of CT in evaluating the patency of portosystemic shunts; accuracy rates ranged from 77 to 100%.29,30 Most errors occur when artifacts from surgical clips or embolization coils obscure a critical region such as the shunt anastomosis. The newer spiral CT techniques, particularly when teamed with three-dimensional reconstruction, should improve the ability to evaluate these shunts noninvasively.
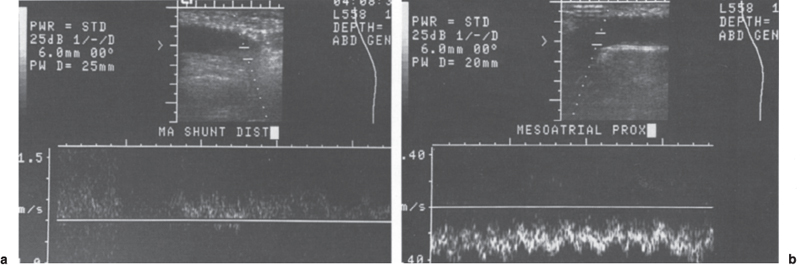
FIGURE 8–3. Duplex Doppler evaluation of mesoatrial shunt. Antegrade flow in the (a) distal and (b) proximal portions of the mesoatrial shunt confirm shunt patency.
Unlike ultrasound and MRI, CT requires the use of intravenous contrast and cannot measure flow velocities. Three-dimensional computer reconstruction combined with helical CT scanning can now provide multiplanar three-dimensional images of blood vessels, shunts, and selected abdominal viscera.31,32 Although no studies are available in which helical CT has been evaluated directly as a modality for imaging portosystemic shunts, this technique should be able to provide even more information than that from conventional dynamic CT.
 Invasive Imaging: Venography and Hemodynamic Assessment
Invasive Imaging: Venography and Hemodynamic Assessment
Following completion of noninvasive imaging, patients typically require venographic evaluation for detailed imaging of the shunt along with hemodynamic evaluation. Initial evaluation of most shunts can be accomplished from the arterial side with a selective splenic or superior mesenteric artery arteriogram performed using digital subtraction techniques. For example, a patent splenorenal or splenopulmonary shunt could be demonstrated by a splenic artery injection. A patent mesoatrial, mesocaval, portocaval, portoatrial, or portopulmonary shunt could be well visualized during the priscolene-enhanced portal venous phase of a superior mesenteric artery arteriogram (Fig. 8–6). This initial study requires only a small volume of contrast and can serve as a road map for selective shunt evaluation. If the shunt is not visualized, it may be assumed that it is either thrombosed or stenotic to the point that it is not supporting any significant blood flow. In most cases, the former will be true; however, in all cases, the next step is selective shunt venography with hemodynamic evaluation. If the shunt was not visualized on the digital subtraction study, a dedicated search for the shunt orifice or anastomosis is performed. If previous arteriographic road maps are available, or if the surgeon has placed a metallic marker such as a clip or a ring adjacent to or on the anastomosis, this task can be greatly simplified.
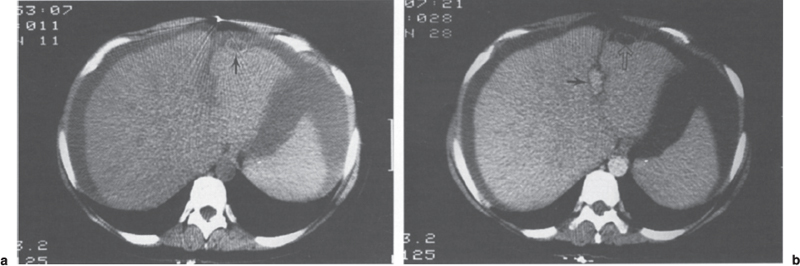
FIGURE 8–4. Dynamic computed tomography evaluation of mesoatrial shunt. (a) An unenhanced scan through the upper portion of the liver demonstrates the shunt (arrow). Note the ascites. (b) Enhanced image following bolus injection of contrast demonstrates opacification of the aorta and falciform ligament (arrow). Note that the mesoatrial shunt (open arrow) does not enhance due to thrombosis. (Reproduced with permission from ref. 28.)
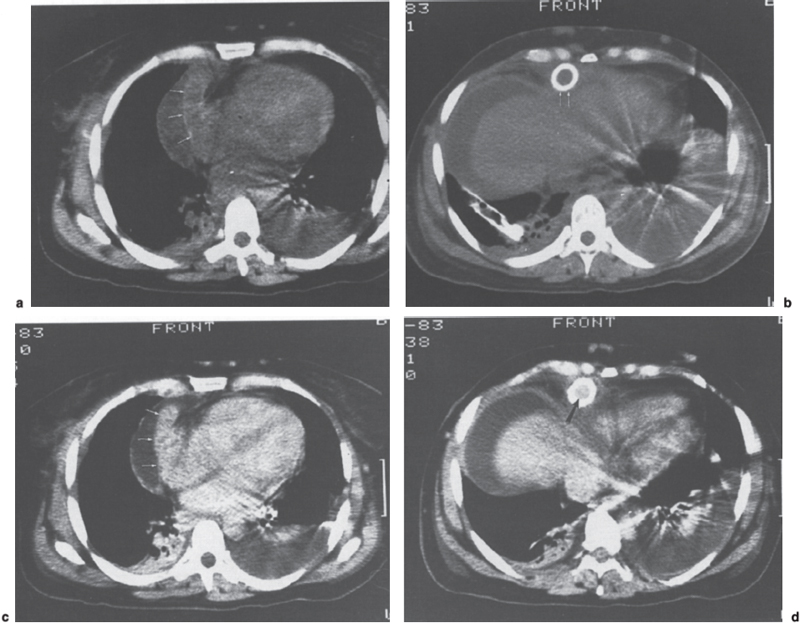
FIGURE 8–5. Dynamic computed tomography (CT) evaluation of mesoatrial shunt. (a) Noncontrast enhanced CT scan at the level of the anastomosis of the shunt (arrows) to the right atrium and (b) through the upper portion of the liver demonstrates the shunt. (c) Image corresponding to (a) following bolus injection of contrast demonstrates complete enhancement of the shunt (arrow) to the same degree as the cardiac chambers. (d) Image corresponding to (b) following bolus injection of contrast demonstrates enhancement of the central lumen of the shunt (arrow). The combined findings in (c) and (d) are compatible with a functioning mesoatrial shunt. (Reproduced with permission from Fishman EK. Surgery. 1985;97:60–64.)
The mesoatrial and portoatrial shunts can best be catheterized from a transjugular or basilic vein approach using a 5F multipurpose or hockey-stick catheter. In patients with a mesocaval, portocaval, or splenorenal shunt, catheterization from a common femoral vein approach is used with a multipurpose, Cobra, or sidewinder type catheter (Figs. 8–7 and 8–8). A Benston (Cook, Bloomington, IN) guidewire can be used for the initial catheterization attempt; but, if it is unsuccessful, a torque-controlled guidewire such as a Wholey (Cook) wire or Terumo (Boston Scientific, Watertown, MA) angle-tipped guidewire is used. In patients with a tight anastomosis, one of the smaller torque-controlled guidewires, such as Road Runner (Boston Scientific), Stubbie (Target Therapeutics, San Jose, CA), or a Flex-J (USCI, Bard, Covington, GA) coronary wire may prove beneficial in obtaining access to the shunt.
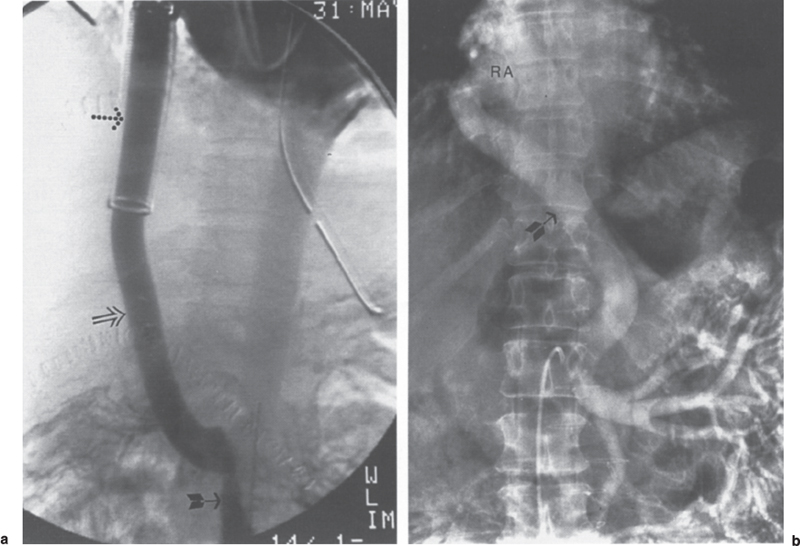
FIGURE 8–6. Patient 1: (a) Portal venous phase of superior mesenteric artery injection demonstrates enhancement of the superior mesenteric vein (arrow) and mesoatrial shunt (open arrow). Note the ribbed portion of the graft (dotted arrow) as it passes through the diaphragm. Patient 2: (b) Portal venous phase of superior mesenteric artery arteriogram demonstrates enhancement of the superior mesenteric vein branches and a nonribbed mesoatrial shunt (arrow). Note the anastomosis of the shunt to the right atrium (RA).
The catheter and guidewire are manipulated across the shunt and into the native vessel lying proximal (i.e., portal vein in patients with a mesocaval or mesoatrial shunt, splenic vein in patients with a splenorenal shunt), and contrast evaluation by hand injection is performed to confirm the catheter’s position and grossly assess the degree of shunt patency. Digital subtraction angiography is performed subsequently to road-map the shunt and its proximal and distal anastomoses and to evaluate for the presence or absence of thrombus. Continuous pressure measurements then are obtained as the catheter (multi-sidehole or nontapered) is pulled back into either the inferior vena cava (IVC) (mesocaval, portocaval shunt) or right atrium (mesoatrial, portoatrial shunt). The catheter may be pulled back over a 0.018-inch guidewire to preserve access in cases of particularly tight stenoses or difficult to access “upstream” portal vessels.
We noted a rather fragile relationship between the pressure gradient across the shunt (anastomoses) and the occurrence of symptoms.13 This “critical gradient” is variable from patient to patient, and gradients of less than 10 mm Hg can result in significant symptomology with return of ascites, abdominal pain, and even variceal hemorrhage.11,13 Even partial correction of these gradients can result in dramatic improvements with resolution of symptoms. We thus treat any reasonable gradient (>3 mm Hg) in the symptomatic patient if no other cause for shunt malfunction or the patient’s symptoms can be determined.
 Percutaneous Intervention
Percutaneous Intervention
Percutaneous Transluminal Angioplasty
Percutaneous transluminal angioplasty (PTA) is an accepted and proven modality for effective treatment of anastomotic and nonthrombotic intrashunt stenoses.13,33–40 Standard catheter and guidewire techniques, as already described, are used to catheterize the shunt. If angioplasty is to follow the diagnostic evaluation, standard guidewire exchange techniques are used to place an exchange-length Rosen 3-mm J (Cook) guidewire or 0.018-inch Stubbie guidewire across the lesion. Because anastomotic strictures tend to be quite fibrotic in nature, a high-pressure balloon is required for most effective dilatations. The size of the balloon to be used should be chosen based on the corrected diameter of the native vessel or shunt as imaged by cut film; however, digital subtraction techniques teamed with a vesselsizing program is more accurate. The structure with the smaller of the two diameters is used to avoid overdilatation and the risk of rupture (Figs. 8–9 and 8–10). We use a balloon of equal diameter or 1 mm larger than the diameter of the shunt or native vessel. If patients are presently on anticoagulants, we do not discontinue treatment before the procedure. Heparin is administered during the procedure (3000–5000 IU) and maintained 24 hours thereafter to obtain an activated partial thromboplastin time ratio (aPTT) 1.5 to 2.0 X control. Patients who initially present with active bleeding are not anticoagulated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



